Health Promotion Perspectives. 14(3):286-296.
doi: 10.34172/hpp.42549
Original Article
Effects of propolis supplementation on prooxidant-antioxidant balance, oxidative stress biomarkers, and body composition in obese patients with NAFLD: A double-blind randomized controlled clinical trial
Hamideh Nazari-Bonab Data curation, Formal analysis, Investigation, Methodology, Project administration, Software, Writing – original draft, 1 
Mahlagha Nikbaf-Shandiz Investigation, Software, 1
Helda Tutunchi Conceptualization, Writing – review & editing, 2 
Mehrangiz Ebrahimi-Mameghani Conceptualization, Data curation, Funding acquisition, Methodology, 3, * 
Author information:
1Student Research Committee, Tabriz University of Medical Sciences, Tabriz, Iran
2Endocrine Research Center, Tabriz University of Medical Sciences, Tabriz, Iran
3Nutrition Research Center, Department of Biochemistry and Diet Therapy, Faculty of Nutrition and Food Sciences, Tabriz University of Medical Sciences, Tabriz, Iran
Abstract
Background:
Oxidative stress is one of the main hits in the pathogenesis of non-alcoholic fatty liver disease (NAFLD). Propolis (PRP), a natural substance made by bees from plant extracts, has been reported to have antioxidant properties. The present clinical trial investigated the effect of Iranian PRP on prooxidant-antioxidant balance (PAB), oxidative stress biomarkers, and body composition in obese patients with NAFLD.
Methods:
In the present double-blind, randomized controlled clinical trial, 44 obese patients with NAFLD were randomly allocated to either Iranian PRP (1500 mg/d) or placebo (1500 mg/d) accompanied by a calorie-restricted diet (CRD) for eight weeks. PAB, oxidative stress biomarkers, and body composition were assessed at baseline and the end of the study.
Results:
There was a significant reduction in PAB levels over the trial in both groups. However, the between-group difference was not significant at the endpoint. At the end of the study, the inter-group comparison showed a significant decrease in serum glutathione peroxidase level in the placebo group compared to the PRP group after adjusting for confounding variables based on models 1 (P=0.027) and 2 (P=0.028). No significant within- or between-group differences in other studied oxidative stress biomarkers were found. Moreover, no between-group differences were observed for body composition and dietary intakes of energy and antioxidant micronutrients.
Conclusion:
Iranian PRP supplementation (1500 mg/d) for eight weeks could prevent the reduction of glutathione peroxidase levels compared to the control group. However, it could not affect other oxidative stress biomarkers, body composition, or dietary intakes of energy and antioxidant micronutrients.
Keywords: Body composition, Liver function tests, Non-alcoholic fatty liver disease, Oxidative stress, Propolis
Copyright and License Information
©2024 The Author(s).
This is an open access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Funding Statement
This study was funded by the ‘Research Vice-Chancellor’ of Tabriz University of Medical Sciences, Tabriz, Iran. This paper is a part of the data obtained from an MSc dissertation submitted to Tabriz University of Medical Sciences (Hamideh Nazari-Bonab).
Introduction
Non-alcoholic fatty liver disease (NAFLD) is defined as a set of conditions ranging from steatosis with no signs of liver cell damage to steatohepatitis with an unremitting inflammatory status to cirrhosis.1-3 The prevalence of NAFLD is estimated to range from 11.2% to 37.2% in the general population and 31% in the Asian population.4 It is the most common chronic liver disease among overweight people.4,5 Overweight and obesity, insulin resistance, dyslipidemia, inflammation, oxidative stress, arterial hypertension, and genetic factors affect not only the onset but also the progression of NAFLD.1,2,5-8
Several studies have investigated the role of oxidative stress as an important factor in the pathology of metabolic conditions such as NAFLD.9,10 According to the “multiple-hit hypothesis”, lipid accumulation in hepatocytes leads to disruption of the mitochondrial antioxidant capacity as well as endoplasmic reticulum stress, exciting fat oxidation pathways.11 Oxidative stress causes an inflammatory response through the positive regulation of highly sensitive C-reactive protein (hs-CRP), proinflammatory cytokines such as interleukin-6 (IL-6), and tumor necrosis factor α (TNF-α).12,13
Although there is no approved treatment for NAFLD, present management is based on lifestyle modification, including healthy dietary patterns and physical activity.14 Additionally, it appears that substances with antioxidant properties could be considered an effective way to control these pathways. Nowadays, there is a great concern about the possible role of natural compounds in preventing liver diseases.15-17 Propolis (PRP) is a natural viscous substance made by bees from plant extracts, flowers, and gums. The combination of PRP with bees’ saliva18-21 has been shown to contain different compositions depending on the plant source and geographical area.22-25 PRP is generally rich in flavonoids, phenolic acids, and terpenes, as well as proteins, sugars, minerals, and vitamins.23,26 A number of properties have been attributed to PRP, e.g., cytotoxicity, antioxidant, free radical scavenging properties, anti-inflammatory, immune stimulant, anti-tumor, liver protection, local anesthetic, antimicrobial, and antiviral activities.18,27-29 The medical benefits of PRP have encouraged studying the chemical compounds and their widespread clinical use in humans.
Various components, particularly flavonoids and polyphenols, are responsible for its antioxidant and biological properties.28 These biological polyphenols exert their effects by chelating ionic metals, preventing the formation of free radicals, and inhibiting the enzymes involved in the initiation of free radical reactions.30,31 Various animal and in vitro studies indicate the positive effect of PRP on improving oxidative stress.32-37 Kismet et al observed that PRP significantly improved malondialdehyde (MDA) and glutathione peroxidase (GPX) levels in rats with NAFLD. Moreover, recent human studies have reported controversial findings regarding the effect of PRP with different dosages and various clinical conditions. Some have illustrated the protective effects of PRP on oxidative stress.38-42 For example, Afsharpour et al reported that PRP administration at 1500 mg in patients with type 2 diabetes mellitus (T2DM) for two months resulted in an improved antioxidant status.38 Likewise, Hesami et al observed a significant improvement in antioxidant status.43 However, Gao et al and Zhao et al did not observe significant results using 900 mg PRP for 16 weeks in patients with T2DM.44,45
Although most studies have investigated antioxidant biomarkers, the prooxidant-antioxidant balance (PAB) method has been introduced. This method is a simple, fast, and inexpensive way to determine the oxidants and antioxidants simultaneously in one single test.46 Only one study by Darvishi et al reported that 8-week supplementation with PRP (500 mg/d) did not affect PAB in patients with breast cancer after chemotherapy.47
The increasing trend in NAFLD prevalence worldwide has limited human studies on the effects of PRP supplementation on oxidative stress assessed by PAB compared with other biomarkers of oxidative stress, particularly in patients with NAFLD, and controversies in findings. The purpose of this study was to examine the effect of PRP supplementation on PAB, oxidative stress, body composition, and liver function in NAFLD patients.
Material and Methods
Setting of the study
This double-blind, placebo-controlled, randomized clinical trial is part of a previously published study.48 The target population was obese patients (n = 44) with NAFLD referred to sub-specialized and specialized clinics of Tabriz University of Medical Sciences from February 2021 to October 2021. Eligible subjects were patients with mild or moderate NAFLD diagnosed with ultrasound, aged 20-50, and a body mass index (BMI) between 30 and 40 kg/m2. NAFLD was diagnosed by a radiologist using the ultrasonography findings of Hamaguchi et al.49 All the patients were given a comprehensive explanation of the study objectives, and written informed consent was obtained from them. The study protocol was approved by the Ethics Committee of the Vice-Chancellor (Ethics code: TBZMED. REC. 1399.942). The protocol was also registered at the Iranian Registry of Clinical Trials (IRCT20100209003320N20).
The exclusion criteria were as follows: suffering other chronic and acute liver diseases such as alcoholic fatty liver, Wilson’s disease, autoimmune liver disease, hepatitis, following a weight loss diet or supplements over the last three months, or taking hepatotoxic, lipid- or glucose-lowering medications and antioxidant supplements in the past three months. Additionally, individuals who were pregnant, lactating, athletes, postmenopause, cigarette smokers, alcohol drinkers, and allergic to PRP or honey products were also excluded.
Sample size
Using power analysis and sample size software (PASS; NCSS, LLC, US), the sample size was estimated at 18 for each group, taking into account the mean change in GPX reported by Afsharpour et al,38 a 95% confidence level, and 90% power. Then, considering a 30% dropout rate, the sample size was increased to 23.
Intervention, randomization, and concealment
An independent assessor assigned patients randomly into one of the two study groups,“PRP” or “Placebo,” in a 1:1 ratio using Random Allocation Software (RAS), in blocks stratified based on BMI (30–34.9 kg/m2vs. 35–39.9 kg/m2), age (20–35 years vs. 36–50 years), and gender (male vs. female). Both patients and investigators were blind to the group allocation until the end of the study. The person who was not involved in the study procedure and determined the eligibility and entry of patients, was responsible for allocation concealment, i.e. the assignments were enclosed in serially numbered, opaque, sealed envelopes with a 3-digit code to each of the treatments.
The patients in the PRP group (n = 23) took 500 mg Iranian PRP capsules (containing 170 mg of poplar-type PRP ethanol extract and 330 mg oat and bee pollen), while those in the placebo group (n = 21) took 500 mg placebo capsules (containing cornstarch and food coloring) three times a day after each meal for 8 weeks. PRP and placebo supplements were similar in size, shape, and color, with a specified code. The dose and duration of the intervention were determined based on the previous study,38 and no side effects were observed with this dose. The adherence was consuming more than 90% of the supplements assessed by counting the capsules returned by the patients. Demographic details and an International Physical Activity Questionnaire (IPAQ) were completed at the beginning and end of the study.
As a result, each participant received a personalized calorie-restricted diet (CRD) based on their resting metabolic rate, calculated using the Mifflin formula, physical activity level, and thermic effect of food (TEE) as 10% of total energy expenditure. To achieve weight loss, the estimated TEE was decreased to -500 kcal. The distribution of macronutrients in the designed CRD was 50% carbohydrates, 30% fat, and 20% protein. After preparing meal plans according to the food-based dietary guidelines for Iranians, a full explanation was given to each patient on how to use food exchange lists and how to replace the foods they did not have access to. A 3-day food record (2 weekdays and one weekend) was obtained at baseline and post-intervention to assess patients’ adherence to the prescribed diets. Dietary intakes were analyzed using Nutritionist IV software (First Databank, San Bruno, CA, USA) modified for Iranian foods. In addition, the patients were monitored for supplement consumption every 2 weeks during the intervention period.
Body composition and BMI
BMI was calculated based on weight (kg) divided by the square of height (m2). Moreover, body composition was assessed using a bioelectric impedance analyzer (Japan, Takara BC-418) by asking patients to remove metal objects and place the soles of their feet and the palms of their hands directly on the electrodes of the device. Also, in order to prevent the device’s accuracy from being disturbed, the patients were required not to exercise for 90-120 minutes before the visit and to drink enough water.
Assessment of dietary intake and physical activity
A 3-day food record (two non-consecutive days of the week and one weekend) was completed at baseline and after 8 weeks for all patients to estimate energy, macronutrients, and dietary antioxidants.
The short form of the International Valid Physical Activity Questionnaire (IPAQ-SF)50 was used to determine physical activity based on metabolic equivalent work minutes per week (MET-min/week).
Study outcomes
The primary outcomes of this trial were changes in oxidative stress biomarkers and PAB levels. The secondary outcomes were changes in body composition, dietary energy intake, and antioxidant micronutrients.
Laboratory assays
A blood sample was taken after 12-14 hours of fasting at the beginning and end of the study. The blood samples were put into tubes with or without EDTA. The EDTA-containing tubes were centrifuged at 3000 rpm for 10 minutes to separate serum, while whole blood samples were applied to determine GPX and superoxide dismutase (SOD) levels after obtaining hemoglobin concentration. Both whole blood and serum samples were stored at -80 °C until analysis. The activity of Cu/Zn-SOD and GPX was quantified using Ransod (Randox Laboratories, Ltd., UK) and Ransel commercial kits (Randox Laboratories, Ltd., UK, BT29 4QY), respectively. MDA concentration was assessed based on reaction with thiobarbituric acid51 using spectrophotometry, whereas serum levels of total antioxidant capacity (TAC) were determined using the Randox TAS kit (Randox Laboratories, Ltd., UK) and spectrophotometry. Serum levels of gamma glutamine transferase (GGT), alanine aminotransferase (ALT), and aspartate aminotransferase (AST) were assessed using the enzymatic method. Moreover, the NAFLD fibrosis score was estimated based on the following formula according to Angulo and colleagues’ study and classified into “low probability of advanced fibrosis” (≤ -1.455), “moderate probability of advanced fibrosis” (−1.455 to 0.676), and “high probability of advanced fibrosis” (≥ 0.676).52
NAFLD fibrosis score = -1.675 + 0.037 × age (years) + 0.094 × BMI (Kg/m2) + 1.13 × impaired fasting glucose (IFG)/diabetes (yes = 1, no = 0) + 0.99 × AST/ALT ratio – 0.013 × platelet × (109/L) – 0.66 × albumin (g/dL).
The serum PAB level was determined using the ELISA technique, following the method introduced and developed by Hamidi-Alamdari and colleagues.46
Statistical analyses
For statistical analysis, the SPSS IBM Statistics version 22 (Armonk, NY: IBM Corp) software and per-protocol principle were used. The Kolmogorov-Smirnov test was used to check the distribution of quantitative data. The data with a normal distribution was presented as mean ± SD, while asymmetric data was reported as median and interquartile range. To compare the variables between the two groups at the beginning of the study, the independent samples t-test and Mann-Whitney U test were applied, while intra-group changes were compared using paired sample t-test and Wilcoxon signed rank test. At the end of the study, ANCOVA to assess inter-group differences after adjusting for confounding variables based on two models: (1) adjusted for baseline values, and (2) adjusted for baseline values as well as energy intake.
Results
Out of the 50 patients who enrolled in the study, three patients in the PRP group (due to COVID-19 infection) and two patients in the placebo group (due to COVID-19 infection and pregnancy) were excluded. Therefore, 44 patients completed the intervention (Figure 1). No side effects or symptoms were reported by the patients following PRP supplementation.
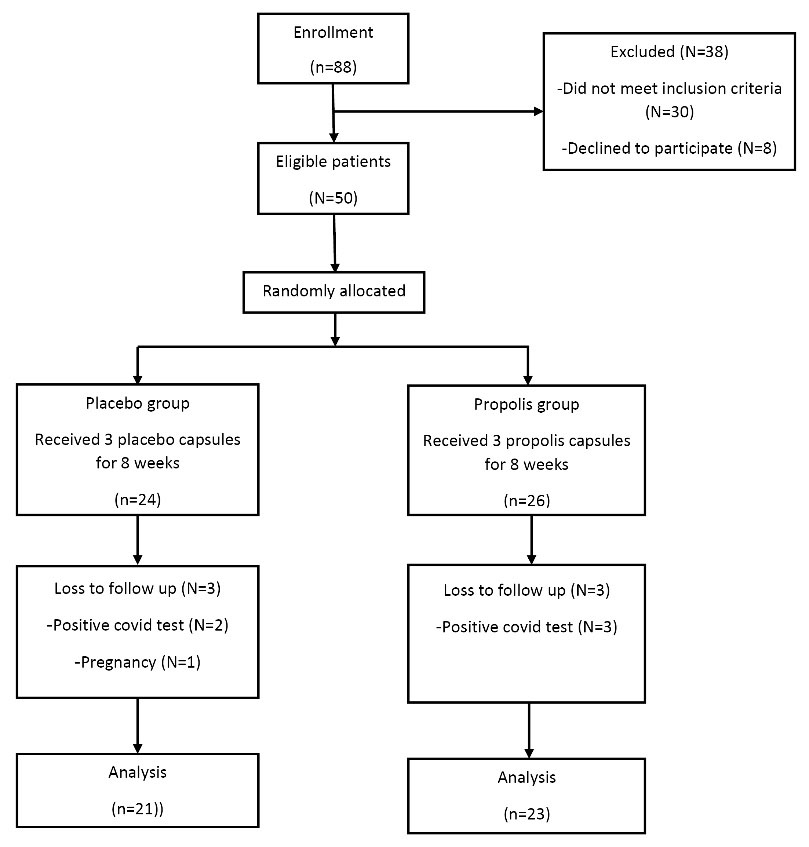
Figure 1.
Trial profile and design
.
Trial profile and design
As shown in Table 1, there were no statistically significant differences between the two groups in terms of body weight, BMI, age, sex, disease severity (in terms of NAFLD grade), fibrosis status, and physical activity level at baseline.
Table 1.
Baseline characteristics of the study participants
|
Variables
|
Propolis
(N=23)
|
Placebo
(N=21)
|
P
|
|
N (%)
|
N (%)
|
| Gender, Female |
15 (65.2) |
13 (61.9) |
0.821b |
| Education |
|
|
|
| High school |
11 (47.8) |
13 (61.9) |
0.349b |
| University degree |
12 (52.2) |
8 (38.1) |
| Physical activity |
|
|
|
| Low |
17 (73.9) |
17 (81.0) |
0.724c |
| Moderate |
6 (26.1) |
4 (19.0) |
| NAFLD severity |
|
|
|
| Grade1 |
7 (30.4) |
9 (42.9) |
0.392b |
| Grade2 |
16 (69.6) |
12 (57.1) |
| Fibrosis status |
|
|
|
| Low |
19 (82.6) |
17 (81.0) |
1.000c |
| Moderate |
4 (17.4) |
4 (19.0) |
|
|
Mean ± SD
|
Mean ± SD
|
|
| Age (years) |
38.52 ± 7.51 |
40.14 ± 9.19 |
0.524a |
| Body weight (kg) |
93.32 ± 15.27 |
89.02 ± 13.22 |
0.327a |
| BMI (kg/m2) |
33.37 ± 4.82 |
33.00 ± 4.18 |
0.792a |
BMI, body mass index.
aIndependent t test. bChi-square. cFisher’s exact test.
There were no significant inter-group differences in dietary intakes of energy, antioxidant micronutrients, or physical activity levels after 8 weeks of intervention (Table 2).
Table 2.
Dietary intakes of energy, antioxidant micronutrients, and physical activity level of the patients over the study
|
Variables
|
Propolis
(N=23)
|
Placebo
(N=21)
|
P
|
| Energy(kcal) |
|
|
|
| Baseline |
1766.52 ± 282.86 |
1706.05 ± 257.99 |
0.464a |
| End |
1650.13 ± 266.694 |
1602.29 ± 296.04 |
0.962c |
| Mean difference (95%) |
-116.39 (-191.03, -41.75) |
-103.76 (-169.49, -38.03) |
|
| P* |
0.004
|
0.004
|
|
| Saturated fatty acids(gr) |
|
| Baseline |
16.69 ± 12 |
17.64 ± 10.83 |
0.769b |
| End |
16.26 ± 8.19 |
16.77 ± 7.40 |
0.926c |
| Mean difference (95%) |
-0.43 (-5.33, 4.47) |
-0.87 (-4.98, 3.25) |
|
| P* |
0.903 |
0.794 |
|
| Polyunsaturated Fatty acid (gr) |
|
| Baseline |
15.06 ± 4.36 |
15.26 ± 4.44 |
0.879a |
| End |
15.00 ± 4.45 |
12.61 ± 5.73 |
0.106c |
| Mean difference (95%) |
-0.06 (-2.48, 2.36) |
-2.65 (0.05, -5.26) |
|
| P* |
0.959 |
0.046
|
|
| Monounsaturated Fatty acid (gr) |
|
| Baseline |
13.21 ± 4.46 |
11.49 ± 5.73 |
0.272a |
| End |
12.82 ± 7.28 |
12.34 ± 5.42 |
0.926c |
| Mean difference (95%) |
-0.39 (-3.76, 2.99) |
0.85 (-1.80, 3.50) |
|
| P* |
0.815 |
0.510 |
|
| Cholesterol (mg) |
|
|
|
| Baseline |
276.68 ± 158.96 |
274.02 ± 152.80 |
0.955a |
| End |
287.18 ± 138.38 |
300.09 ± 130.50 |
0.640c |
| Mean difference (95%) |
10.49 (-44.13, 65.11) |
26.07 (-25.60, 77.74) |
|
| P* |
0.815 |
0.305 |
|
| Vitamin A (mg) |
|
|
|
| Baseline |
841.74 ± 861.93 |
703.68 ± 448.89 |
0.235b |
| End |
603.99 ± 392.85 |
707.40 ± 406.38 |
0.556d |
| Mean difference (95%) |
-237.75 (-626.44, 150.93) |
3.71 (-306.98, 314.41) |
|
| P** |
0.855 |
0.741 |
|
| Vitamin C (mg) |
|
|
|
| Baseline |
87.15 ± 63.34 |
65.70 ± 42.20 |
0.254b |
| End |
74.53 ± 46.87 |
57.73 ± 24.20 |
0.668d |
| Mean difference (95%) |
-12.62 (-35.08, 9.84) |
-7.96 (-30.05, -14.12) |
|
| P** |
0.201 |
0.876 |
|
| Vitamin E (mg) |
|
|
|
| Baseline |
7.51 ± 9.72 |
5.32 ± 6.49 |
0.391b |
| End |
7.15 ± 8.04 |
4.68 ± 3.95 |
0.746d |
| Mean difference (95%) |
-0.36 (-2.14, -1.41) |
-0.64 (-2.17, 0.89) |
|
| P** |
0.394 |
0.808 |
|
| Selenium (mg) |
|
|
|
| Baseline |
0.09 ± 0.03 |
0.08 ± 0.02 |
0.238a |
| End |
0.07 ± 0.03 |
0.08 ± 0.03 |
0.113c |
| Mean difference (95%) |
-0.01 (-0.031, 0.004) |
-0.01 (-0.016, 0.003) |
|
| P* |
0.116 |
0.190 |
|
| Zinc (mg) |
|
|
|
| Baseline |
7.9 ± 2.46 |
7.45 ± 2.09 |
0.879b |
| End |
7.64 ± 2.6 |
8.21 ± 2 |
0.481d |
| Mean difference (95%) |
-0.27 (-1.37, 0.83) |
0.76 (-0.57, 2.1) |
|
| P** |
0.362 |
0.476 |
|
| Copper (mg) |
|
|
|
| Baseline |
1.1 ± 0.66 |
0.94 ± 0.23 |
0.488b |
| End |
0.88 ± 0.22 |
0.84 ± 0.33 |
0.253d |
| Mean difference (95%) |
-0.21 (-0.52, 0.10) |
-0.10 (-0.23, 0.04) |
|
| P** |
0.191 |
0.164 |
|
| PA (METs) |
|
|
|
| Baseline |
444.70 ± 180.37 |
427.07 ± 173.98 |
0.744a |
| End |
437.22 ± 200.28 |
422.71 ± 173.28 |
0.908c |
| Mean difference (95%) |
-7.48 (-41.23, 26.57) |
-4.36 (-35.91, 27.20) |
|
| P* |
0.653 |
0.776 |
|
PA, physical activity; METs, metabolic equivalents (MET-min/week).
Mean (SD) and mean difference (95% CI) are presented for data. P < 0.05 is defined as a significant level (Bold numbers).
*Paired samples t-test
** Wilcoxon signed-rank test
a Independent sample t-test.
b Mann-Whitney test
c ANCOVA test (adjusted for baseline values).
d Quantile regression (adjusted for baseline values).
Table 3demonstrates serum levels of PAB as well as oxidative stress biomarkers at baseline and at the end of the study. Results showed that there was a significant reduction in PAB levels over the course of the trial in both groups. However, the inter-group difference was not significant at the endpoint. At the end of the study, the inter-group comparison showed a significant decrease in serum GPX level in the placebo group compared to the PRP group after adjusting for confounding variables based on models 1 (P = 0.027) and 2 (P = 0.028). No significant intra- or inter-group differences in other studied oxidative stress biomarkers, including MDA, SOD, and TAC, were found. Although the serum levels of ALT (P = 0.002), AST (P = 0.018),and GGT (P < 0.001) decreased significantly in the PRP group, between-group differences were not significant for these parameters (data are reported in our previously published article).48 However, the NAFLD fibrosis score improved significantly in the PRP group compared to the placebo group after adjusting for confounding factors (P = 0.021).
Table 3.
Antioxidant and oxidative stress markers and body composition of the study participants throughout the study
| Variables |
Propolis
(N=23)
|
Placebo
(N=21)
|
P
|
| PAB (HK) |
|
|
|
| Baseline |
96.63 ± 27.01 |
73.61 ± 24.45 |
23.02 (7.29, 38.74), 0.05a |
| End |
75.29 ± 22 |
59.43 ± 17.07 |
-9.23 (-21.68, 3.21), 0.142b, 0.147c |
| Mean difference (95%) |
-21.34 (-34.12, -8.52) |
-14.18 (-23.85, -4.5) |
|
| P* |
0.002
|
0.006
|
|
| TAC (mmol/l) |
|
|
|
| Baseline |
1.51 ± 0.28 |
1.53 ± 0.35 |
0.02 (-018, 0.21), 0.868a |
| End |
1.48 ± 0.32 |
1.49 ± 0.35 |
0.001(-0.149, 0.151), 0.993b, 0.995c |
| Mean difference (95%) |
-0.038 (-0.16, 0.08) |
-0.04 (-0.15, 0.07) |
|
| P* |
0.516 |
0.419 |
|
| MDA (nmol/ml) |
|
|
|
| Baseline |
1.88 ± 0.53 |
1.75 ± 0.48 |
0.02 (-0.35,0.39), 0.935a |
| End |
1.83 ± 0.60 |
2.07 ± 0.79 |
-0.23 (-0.64, 0.19), 0.089b, 0.095c |
| Mean difference (95%) |
-0.04 (-0.38, 0.29) |
0.17 (-0.17, 0.52) |
|
| P* |
0.791 |
0.313 |
|
| GPX (U/gHb) |
|
|
|
| Baseline |
52.13 ± 14.43 |
53.99 ± 12.73 |
-1.86 (-10.17, 6.45), 0.654a |
| End |
48.40 ± 17.12 |
39.61 ± 11.66 |
-9.57 (-17.99, -1.15), 0.027
b
, 0.028
c
|
| Mean difference (95%) |
-3.73 (-11.16, 3.71) |
-14.38 (-20.76, -8.00) |
|
| P* |
0.310 |
<0.001
|
|
| SOD (U/gHb) |
|
|
|
| Baseline |
1278.19 ± 238.83 |
1398.54 ± 197.05 |
-91.40 ( -210.55, 27.76), 0.077a |
| End |
1357.47 ± 202.48 |
1333.04 ± 147.83 |
-39.26 (-150.72, 72.19), 0.481b, 0.492c |
| Mean difference (95%) |
79.28 (-31.84, 190.40) |
-65.49 (-150.50, 19.51) |
|
| P* |
0.153 |
0.124 |
|
| BMI (kg/m2) |
|
|
|
| Baseline |
33.37 ± 4.82 |
33.00 ± 4.18 |
0.36 (-2.40, 3.12), 0.792a |
| End |
32.03 ± 5.27 |
31.88 ± 4.48 |
0.24 (-0.40, 0.88), 0.460b, 0.503c |
| Mean difference (95%) |
-1.34 (-1.88, -0.80) |
-1.12 (-1.51, -0.74) |
|
| P* |
<0.001
|
<0.001
|
|
| FFM (kg) |
|
|
|
| Baseline |
62.54 ± 11 |
60.50 ± 9.45 |
2.04 (-4.23, 8.31), 0.514a |
| End |
61.45 ± 11.66 |
58.79 ± 8.84 |
-0.64 (-2.01, 0.72), 0.346b, 0.329c |
| Mean difference (95%) |
-1.09 (-2.10, -0.08) |
-1.79 (-2.64, -0.78) |
|
| P* |
0.036
|
0.001
|
|
| FM (kg) |
|
|
|
| Baseline |
30.43 ± 9.31 |
27.96 ± 8.78 |
2.47 (-3.05, 7.99), 0.372a |
| End |
29.02 ± 11.19 |
26.91 ± 9.54 |
0.55 (-1.76, 2.86), 0.633b, 0.696c |
| Mean difference (95%) |
-1.41 (-3.55, 0.72) |
-1.05 (-1.85, -0.25) |
|
| P* |
0.184 |
0.012
|
|
| FFM (%) |
|
|
|
| Baseline |
67.21 ± 7.03 |
68.17 ± 6.77 |
-0.96(-5.17, 3.25), 0.648a |
| End |
68.84 ± 7.31 |
68.77 ± 7.09 |
-1.02 (-2.39, 0.35), 0.141b, 0.158c |
| Mean difference (95%) |
1.63 (0.45, 2.80) |
0.60 (-0.08, 1.28) |
|
| P* |
0.009
|
0.082 |
|
| FM (%) |
|
|
|
| Baseline |
32.50 ± 7.24 |
31.40 ± 6.84 |
1.09 (-3.21, 5.39), 0.611a |
| End |
31.23 ± 7.60 |
31.00 ± 7.19 |
0.86 (-0.60, 2.37), 0.241b, 0.272c |
| Mean difference (95%) |
-1.26 (-2.51, -0.01) |
-0.40 (-1.11, 0.31) |
|
| P* |
0.048
|
0.256 |
|
| BW (%) |
|
|
|
| Baseline |
48.60 ± 5.37 |
43.50 ± 6.94 |
-0.76 (-4.00, 2.48), 0.505a |
| End |
49.62 ± 5.61 |
42.15 ± 6.36 |
-0.91 (-1.99, 0.17), 0.097b, 0.102c |
| Mean difference (95%) |
1.02 (0.08, 1.97) |
-1.36 (-2.04, -0.67) |
|
| P* |
0.035
|
0.001
|
|
| NAFLD Fibrosis score |
|
|
|
| Baseline |
-2.21 ± 0.87 |
-2.21 ± 1.00 |
-0.003 (-0.57, 0.56), 0.990a |
| End |
-2.47 ± 0.68 |
-1.97 ± 0.90 |
0.50 (0.09, 0.91), 0.021
b
, 0.023
c
|
| Mean difference (95%) |
-0.25 (-0.59, 0.09) |
0.24 (-0.15, 0.63) |
|
| P* |
0.136 |
0.212 |
|
MDA, malondialdehyde; TAC, Total antioxidant capacity; SOD, superoxide dismutase; GPX, Glutathione peroxidase; PAB, prooxidant-antioxidant balance; BMI, Body mass index; FFM, Fat free mass; FM, Fat mass; BW, Body water; NAFLD, Non-alcoholic fatty liver disease.
Values are reported as mean ± standard deviation. P <0.05 is defined as a significant level (Bold numbers).
* Paired samples t-test.
a Independent sample t-test.
c ANCOVA test, adjusted for baseline values (model 1).
b ANCOVA test, adjusted for baseline values and energy changes (model 2).
Although BMI significantly decreased in both groups, the between-group difference was not statistically significant after adjusting for baseline values and energy intake. We found a significant reduction in fat mass (FM) (%) and a significant increase in fat-free mass (FFM) (%) in the PRP group (P = 0.048 and P = 0.009, respectively). However, no significant between-group differences were observed for these parameters at the end of the study. Moreover, although there was a significant increase in total body water (%) in the PRP group (P = 0.035) and a significant reduction in the placebo group (P = 0.001), the between-group differences did not reach statistical significance, even after adjusting for the confounders.
Discussion
As far as we know, the present clinical trial is the first study to examine the effects of PRP supplementation along with a weight loss diet on oxidative stress biomarkers in obese patients with NAFLD, particularly using the PAB assay.
In our study, an individualized weight-loss diet was administered to each patient and followed for 8 weeks. There were no significant differences between the groups in terms of dietary intake of energy and antioxidant micronutrients, as well as physical activity level, after 8 weeks of intervention (Table 2). As a result, these factors were not deemed confounding. However, we adjusted all study outcomes for baseline variables and changes in energy intake. Moreover, although both groups showed significant improvements in anthropometric measures and body composition, no inter-group differences were found for these parameters after adjusting for the confounders at the end of the study (Table 3), which is in line with the results of some previous studies. For example, Soleimani et al42,53 investigated the effects of 500 and 900 mg of PRP supplementation for 4 weeks in athletes and NAFLD patients. They reported that there was no significant effect on weight, FM, or FFM. Indeed, the results of a recent meta-analysis confirmed that PRP does not impact body weight or BMI.54 However, Koya-Miyata et al examined the effect of PRP in a mouse obesity model induced by a high-fat diet. They found that the administration of PRP (with a dose of 5 and 50 mg twice a day) compared to the control group for 10 days led to a decrease in body weight gain and weight of visceral adipose tissue by decreasing mRNA expression related to fatty acid biosynthesis, which includes sterol regulatory element binding protein, fatty acid synthase, and acetyl-CoA carboxylase in the PRP-treated mice.55 We failed to find improvements in anthropometric measures and body composition, possibly due to the relatively short duration of our study.
Regarding oxidative stress biomarkers, results revealed that GPX change in the PRP group was less than in the placebo group (-3.73 U/gHb vs. -14.38 U/gHb), and the inter-group difference was found statistically significant after adjusting for both baseline values and change in energy intake (P= 0.027) (Table 3). In addition, serum MDA level, as a simple indicator of fat oxidation,56 decreased in the PRP group compared with an increase in the placebo group, while SOD increased in the PRP group compared with a decrease in the placebo group. However, both intra- and inter-group changes were not statistically significant. Serum PAB level showed a greater reduction in the PRP group than in the placebo group (-21.34 HK vs. -14.18 HK), but the difference was not significant (P= 0.142). In some animal studies, the positive effect of PRP on oxidative stress biomarkers was reported.35,37,57 Kismet et al showed that different doses (100 and 200 mg/kg) of PRP for two weeks in rats with NAFLD significantly improved MDA and GPX levels.35 Other studies investigating the effect of PRP supplementation (900 mg/d) in patients with T2DM for 18 weeks reported no significant effect on SOD, GPX, and MDA concentrations.44,45 However, PRP supplementation (1500 mg/d for 8 weeks) in T2DM patients had a significant increase in serum TAC, SOD, and GPX.38 PRP administration has also been reported to improve other oxidative stress biomarkers, such as catalase (CAT) and oxidized LDL, in T2DM patients.43 In the study by Darvishi et al, 250 mg PRP supplementation a day for a week before chemotherapy led to a significant improvement in oxidant/antioxidant balance compared to baseline.47 Indeed, the results of our recent meta-analysis of controlled clinical trials confirmed that PRP does not influence SOD and MDA but has a beneficial effect on GSH, GPX, and TAC levels.58 The antioxidant property of PRP is mainly related to the composition of flavonoids and polyphenols.59 It varies according to geographical location, source, and season of collection,60,61 which may explain the discrepancy between the results of different studies. Flavonoids inhibit radical species, protect antioxidant defense, and suppress reactive oxygen species (ROS) formation by inhibiting enzymes and chelating elements involved in the production of free radicals.62 Furthermore, PRP prevents oxidative stress by affecting the nuclear factor erythroid 2-related factor 2-antioxidant response element (Nrf2-ARE) pathway and increasing antioxidant response element (ARE) activation63 which is a main endogenous cellular defense mechanism against oxidative stress.64 Moreover, ethanolic extracts of Baccharis PRP, mainly containing artepillin C, camphoride, and chlorogenic acid, enhance nuclear translocation of Nrf2 and induce expression of thioredoxin reductase-1 (TRX-1), glutamate-cysteine ligase modifier subunit (GCLM), and heme oxygenase-1 (HO-1) in RAW 264.7 cells.65
Although we observed significant reductions in serum liver enzymes, including ALT, AST, and GGT, between-group differences were not statistically significant. In addition, the AST/ALT ratio increased significantly in the placebo group, with no significant change in the PRP group. However, inter-group differences were not statistically significant (data are shown in our previously published article).48 Similarly, a previous study evaluating the efficacy of PRP (500 mg/d for 4 months) on hepatic steatosis and fibrosis in patients with NAFLD reported that PRP had no effect on serum levels of AST, ALT, GGT, and alkaline phosphatase in these patients.53 Nevertheless, some clinical trials with different designs have reported the beneficial effects of PRP supplementation on serum liver enzymes. For example, Silveira et al found that PRP administration (500 mg/d for 12 months) could prevent the increase of serum ALT in patients with chronic kidney disease.66 The discrepancy between the findings of studies may be due to differences in dosages of PRP, duration of the supplementation, and differences in clinical conditions. In the present study, we found that PRP supplementation led to a significant improvement in the NAFLD fibrosis score. Similarly, Kismet et al reported that PRP has positive effects on the histopathological and biochemical parameters of NAFLD, and these effects are related to the antioxidant and anti-inflammatory effects of PRP.35 Our finding was also in agreement with that of Soleimani et al, reporting the protective effects of PRP (50 mg twice daily for 4 months) on hepatic steatosis and fibrosis.53
To the best of our knowledge, this trial appears to be the first study investigating PRP effects on oxidative stress biomarkers in patients with NAFLD. As an effective strategy for treating NAFLD, our participants received a CRD and were monitored every two weeks. However, the short duration of the study and the lack of a liver biopsy for NAFLD confirmation because of ethical problems are considered limitations of the present study.
Conclusion
Consuming Iranian PRP supplementation (1500 mg/d) for eight weeks could prevent the reduction of GPX levels compared to the control group. However, it could not affect other oxidative stress biomarkers, body composition, or dietary intakes of energy and antioxidant micronutrients. Further clinical trials with larger sample sizes and longer durations are recommended.
Acknowledgments
We thank the ‘Research Vice-Chancellor’ of Tabriz University of Medical Sciences, Tabriz, Iran for financial support. Also we sincerely thank the patients who cooperated in this study.
Competing Interests
The authors declare that they have no competing interests
Ethical Approval
All patients completed and signed the consent form after knowing the study objectives and protocol. This trial was approved by the Ethics Committee of the Research vice-chancellor (ethical code: TBZMED. REC.1399.942) as well as registered on the website www.irct.ir with the code (IRCT20100209003320N20).
References
- Maciejewska D, Stachowska E. Non-alcoholic fatty liver disease (NAFLD)–epidemic of the XXI century. Postępy Hig Med Dośw 2018; 72:659-70. doi: 10.5604/01.3001.0012.2054 [Crossref] [ Google Scholar]
- Vernon G, Baranova A, Younossi ZM. Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther 2011; 34(3):274-85. doi: 10.1111/j.1365-2036.2011.04724.x [Crossref] [ Google Scholar]
- Tutunchi H, Naeini F, Ebrahimi-Mameghani M, Mobasseri M, Naghshi S, Ostadrahimi A. The association of the steatosis severity, NAFLD fibrosis score and FIB-4 index with atherogenic dyslipidaemia in adult patients with NAFLD: a cross-sectional study. Int J Clin Pract 2021; 75(6):e14131. doi: 10.1111/ijcp.14131 [Crossref] [ Google Scholar]
- Dufour JF, Scherer R, Balp MM, McKenna SJ, Janssens N, Lopez P. The global epidemiology of nonalcoholic steatohepatitis (NASH) and associated risk factors–a targeted literature review. Endocr Metab Sci 2021; 3:100089. doi: 10.1016/j.endmts.2021.100089 [Crossref] [ Google Scholar]
- Tutunchi H, Saghafi-Asl M, Ebrahimi-Mameghani M, Ostadrahimi A. Food insecurity and lipid profile abnormalities are associated with an increased risk of nonalcoholic fatty liver disease (NAFLD): a case-control study. Ecol Food Nutr 2021; 60(4):508-24. doi: 10.1080/03670244.2021.1875453 [Crossref] [ Google Scholar]
- Tilg H, Moschen AR. Evolution of inflammation in nonalcoholic fatty liver disease: the multiple parallel hits hypothesis. Hepatology 2010; 52(5):1836-46. doi: 10.1002/hep.24001 [Crossref] [ Google Scholar]
- Tutunchi H, Mobasseri M, Aghamohammadzadeh N, Hooshyar J, Naeini F, Najafipour F. Serum neuregulin 4 (NRG-4) level and non-alcoholic fatty liver disease (NAFLD): a case-control study. Int J Clin Pract 2021; 75(10):e14555. doi: 10.1111/ijcp.14555 [Crossref] [ Google Scholar]
- Tutunchi H, Naeini F, Mobasseri M, Ostadrahimi A. Triglyceride glucose (TyG) index and the progression of liver fibrosis: a cross-sectional study. Clin Nutr ESPEN 2021; 44:483-7. doi: 10.1016/j.clnesp.2021.04.025 [Crossref] [ Google Scholar]
- Gabbia D, Cannella L, De Martin S. The role of oxidative stress in NAFLD-NASH-HCC transition-focus on NADPH oxidases. Biomedicines 2021; 9(6):687. doi: 10.3390/biomedicines9060687 [Crossref] [ Google Scholar]
- Arroyave-Ospina JC, Wu Z, Geng Y, Moshage H. Role of oxidative stress in the pathogenesis of non-alcoholic fatty liver disease: implications for prevention and therapy. Antioxidants (Basel) 2021; 10(2):174. doi: 10.3390/antiox10020174 [Crossref] [ Google Scholar]
- Buzzetti E, Pinzani M, Tsochatzis EA. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD). Metabolism 2016; 65(8):1038-48. doi: 10.1016/j.metabol.2015.12.012 [Crossref] [ Google Scholar]
- Jou J, Choi SS, Diehl AM. Mechanisms of disease progression in nonalcoholic fatty liver disease. Semin Liver Dis 2008; 28(4):370-9. doi: 10.1055/s-0028-1091981 [Crossref] [ Google Scholar]
- Marra F, Gastaldelli A, Svegliati Baroni G, Tell G, Tiribelli C. Molecular basis and mechanisms of progression of non-alcoholic steatohepatitis. Trends Mol Med 2008; 14(2):72-81. doi: 10.1016/j.molmed.2007.12.003 [Crossref] [ Google Scholar]
- National Guideline Centre (UK). Non-Alcoholic Fatty Liver Disease: Assessment and Management. London: National Institute for Health and Care Excellence (NICE); 2016.
- Del Prete A, Scalera A, Iadevaia MD, Miranda A, Zulli C, Gaeta L. Herbal products: benefits, limits, and applications in chronic liver disease. Evid Based Complement Alternat Med 2012; 2012:837939. doi: 10.1155/2012/837939 [Crossref] [ Google Scholar]
- Stickel F, Schuppan D. Herbal medicine in the treatment of liver diseases. Dig Liver Dis 2007; 39(4):293-304. doi: 10.1016/j.dld.2006.11.004 [Crossref] [ Google Scholar]
- Ebrahimi-Mameghani M, Jamali H, Mahdavi R, Kakaei F, Abedi R, Kabir-Mamdooh B. Conjugated linoleic acid improves glycemic response, lipid profile, and oxidative stress in obese patients with non-alcoholic fatty liver disease: a randomized controlled clinical trial. Croat Med J 2016; 57(4):331-42. doi: 10.3325/cmj.2016.57.331 [Crossref] [ Google Scholar]
- Toreti VC, Sato HH, Pastore GM, Park YK. Recent progress of propolis for its biological and chemical compositions and its botanical origin. Evid Based Complement Alternat Med 2013; 2013:697390. doi: 10.1155/2013/697390 [Crossref] [ Google Scholar]
- Búfalo MC, Candeias JM, Sforcin JM. In vitro cytotoxic effect of Brazilian green propolis on human laryngeal epidermoid carcinoma (HEp-2) cells. Evid Based Complement Alternat Med 2009; 6(4):483-7. doi: 10.1093/ecam/nem147 [Crossref] [ Google Scholar]
- Castaldo S, Capasso F. Propolis, an old remedy used in modern medicine. Fitoterapia 2002; 73 Suppl 1:S1-6. doi: 10.1016/s0367-326x(02)00185-5 [Crossref] [ Google Scholar]
- Fuliang HU, Hepburn HR, Xuan H, Chen M, Daya S, Radloff SE. Effects of propolis on blood glucose, blood lipid and free radicals in rats with diabetes mellitus. Pharmacol Res 2005; 51(2):147-52. doi: 10.1016/j.phrs.2004.06.011 [Crossref] [ Google Scholar]
- Lemos M, de Barros MP, Sousa JP, da Silva Filho AA, Bastos JK, de Andrade SF. Baccharis dracunculifolia, the main botanical source of Brazilian green propolis, displays antiulcer activity. J Pharm Pharmacol 2007; 59(4):603-8. doi: 10.1211/jpp.59.4.0017 [Crossref] [ Google Scholar]
- Huang S, Zhang CP, Wang K, Li GQ, Hu FL. Recent advances in the chemical composition of propolis. Molecules 2014; 19(12):19610-32. doi: 10.3390/molecules191219610 [Crossref] [ Google Scholar]
- Trusheva B, Popova M, Bankova V, Simova S, Marcucci MC, Miorin PL. Bioactive constituents of Brazilian red propolis. Evid Based Complement Alternat Med 2006; 3(2):249-54. doi: 10.1093/ecam/nel006 [Crossref] [ Google Scholar]
- Salatino A, Fernandes-Silva CC, Righi AA, Salatino ML. Propolis research and the chemistry of plant products. Nat Prod Rep 2011; 28(5):925-36. doi: 10.1039/c0np00072h [Crossref] [ Google Scholar]
- Volpi N. Separation of flavonoids and phenolic acids from propolis by capillary zone electrophoresis. Electrophoresis 2004; 25(12):1872-8. doi: 10.1002/elps.200405949 [Crossref] [ Google Scholar]
- Bankova VS, de Castro SL, Marcucci MC. Propolis: recent advances in chemistry and plant origin. Apidologie 2000; 31(1):3-15. doi: 10.1051/apido:2000102 [Crossref] [ Google Scholar]
- Gregoris E, Stevanato R. Correlations between polyphenolic composition and antioxidant activity of Venetian propolis. Food Chem Toxicol 2010; 48(1):76-82. doi: 10.1016/j.fct.2009.09.018 [Crossref] [ Google Scholar]
- Banskota AH, Tezuka Y, Adnyana IK, Midorikawa K, Matsushige K, Message D. Cytotoxic, hepatoprotective and free radical scavenging effects of propolis from Brazil, Peru, the Netherlands and China. J Ethnopharmacol 2000; 72(1-2):239-46. doi: 10.1016/s0378-8741(00)00252-x [Crossref] [ Google Scholar]
- Russo A, Acquaviva R, Campisi A, Sorrenti V, Di Giacomo C, Virgata G. Bioflavonoids as antiradicals, antioxidants and DNA cleavage protectors. Cell Biol Toxicol 2000; 16(2):91-8. doi: 10.1023/a:1007685909018 [Crossref] [ Google Scholar]
- Kurek-Górecka A, Rzepecka-Stojko A, Górecki M, Stojko J, Sosada M, Swierczek-Zieba G. Structure and antioxidant activity of polyphenols derived from propolis. Molecules 2013; 19(1):78-101. doi: 10.3390/molecules19010078 [Crossref] [ Google Scholar]
- Singla S, Kumar NR, Kaur J. In vivo studies on the protective effect of propolis on doxorubicin-induced toxicity in liver of male rats. Toxicol Int 2014; 21(2):191-5. doi: 10.4103/0971-6580.139808 [Crossref] [ Google Scholar]
- Hotta S, Uchiyama S, Ichihara K. Brazilian red propolis extract enhances expression of antioxidant enzyme genes in vitro and in vivo. Biosci Biotechnol Biochem 2020; 84(9):1820-30. doi: 10.1080/09168451.2020.1773756 [Crossref] [ Google Scholar]
- Misir S, Aliyazicioğlu Y, Demir S, Turan I, Yaman S, Değer O. Antioxidant properties and protective effect of Turkish propolis on t-BHP-induced oxidative stress in foreskin fibroblast cells. Indian J Pharm Educ Res 2018; 52(1):94-100. doi: 10.5530/ijper.52.1.11 [Crossref] [ Google Scholar]
- Kismet K, Ozcan C, Kuru S, Gencay Celemli O, Celepli P, Senes M. Does propolis have any effect on non-alcoholic fatty liver disease?. Biomed Pharmacother 2017; 90:863-71. doi: 10.1016/j.biopha.2017.04.062 [Crossref] [ Google Scholar]
- Mahmoud AR, Shalaby NM. Evaluation of hepatoprotective effect of propolis against sub-chronic diazinon induced hepatotoxicity. Egypt J Forensic Sci Appl Toxicol 2018; 18(4):53-67. doi: 10.21608/ejfsat.2018.5216.1025 [Crossref] [ Google Scholar]
- Arabameri A, Sameni H, Bandegi A. The effects of propolis extract on ovarian tissue and oxidative stress in rats with maternal separation stress. Int J Reprod Biomed 2017; 15(8):509-20. doi: 10.29252/ijrm.15.8.509 [Crossref] [ Google Scholar]
- Afsharpour F, Javadi M, Hashemipour S, Koushan Y, Khadem Haghighian H. Propolis supplementation improves glycemic and antioxidant status in patients with type 2 diabetes: a randomized, double-blind, placebo-controlled study. Complement Ther Med 2019; 43:283-8. doi: 10.1016/j.ctim.2019.03.001 [Crossref] [ Google Scholar]
- Ebeid SA, Abd El Moneim NA, El-Benhawy SA, Hussain NG, Hussain MI. Assessment of the radioprotective effect of propolis in breast cancer patients undergoing radiotherapy. New perspective for an old honey bee product. J Radiat Res Appl Sci 2016; 9(4):431-40. doi: 10.1016/j.jrras.2016.06.001 [Crossref] [ Google Scholar]
- Gholaminejad F, Javadi M, Karami AA, Alizadeh F, Kavianpour M, Khadem Haghighian H. Propolis supplementation effects on semen parameters, oxidative stress, inflammatory biomarkers and reproductive hormones in infertile men with asthenozoospermia; a randomized clinical trial. Int J Med Lab 2019; 6(1):21-32. doi: 10.18502/ijml.v6i1.504 [Crossref] [ Google Scholar]
- Mujica V, Orrego R, Pérez J, Romero P, Ovalle P, Zúñiga-Hernández J. The role of propolis in oxidative stress and lipid metabolism: a randomized controlled trial. Evid Based Complement Alternat Med 2017; 2017:4272940. doi: 10.1155/2017/4272940 [Crossref] [ Google Scholar]
- Soleimani D, Miryan M, Hadi V, Gholizadeh Navashenaq J, Moludi J, Sayedi SM. Effect of propolis supplementation on athletic performance, body composition, inflammation, and oxidative stress following intense exercise: a triple-blind randomized clinical trial. Food Sci Nutr 2021; 9(7):3631-40. doi: 10.1002/fsn3.2319 [Crossref] [ Google Scholar]
- Hesami S, Hashemipour S, Shiri-Shahsavar MR, Koushan Y, Khadem Haghighian H. Administration of Iranian propolis attenuates oxidative stress and blood glucose in type II diabetic patients: a randomized, double-blind, placebo-controlled, clinical trial. Caspian J Intern Med 2019; 10(1):48-54. doi: 10.22088/cjim.10.1.48 [Crossref] [ Google Scholar]
- Gao W, Pu L, Wei J, Yao Z, Wang Y, Shi T. Serum antioxidant parameters are significantly increased in patients with type 2 diabetes mellitus after consumption of Chinese propolis: a randomized controlled trial based on fasting serum glucose level. Diabetes Ther 2018; 9(1):101-11. doi: 10.1007/s13300-017-0341-9 [Crossref] [ Google Scholar]
- Zhao L, Pu L, Wei J, Li J, Wu J, Xin Z. Brazilian green propolis improves antioxidant function in patients with type 2 diabetes mellitus. Int J Environ Res Public Health 2016; 13(5). doi: 10.3390/ijerph13050498 [Crossref]
- Hamidi Alamdari D, Paletas K, Pegiou T, Sarigianni M, Befani C, Koliakos G. A novel assay for the evaluation of the prooxidant-antioxidant balance, before and after antioxidant vitamin administration in type II diabetes patients. Clin Biochem 2007; 40(3-4):248-54. doi: 10.1016/j.clinbiochem.2006.10.017 [Crossref] [ Google Scholar]
- Darvishi N, Yousefinejad V, Akbari ME, Abdi M, Moradi N, Darvishi S. Antioxidant and anti-inflammatory effects of oral propolis in patients with breast cancer treated with chemotherapy: a randomized controlled trial. J Herb Med 2020; 23:100385. doi: 10.1016/j.hermed.2020.100385 [Crossref] [ Google Scholar]
- Nikbaf-Shandiz M, Tutunchi H, Khoshbaten M, Nazari-Bonab H, Ebrahimi-Mameghani M. Propolis supplementation in obese patients with non-alcoholic fatty liver disease: effects on glucose homeostasis, lipid profile, liver function, anthropometric indices and meta-inflammation. Food Funct 2022; 13(22):11568-78. doi: 10.1039/d2fo01280d [Crossref] [ Google Scholar]
- Hamaguchi M, Kojima T, Itoh Y, Harano Y, Fujii K, Nakajima T. The severity of ultrasonographic findings in nonalcoholic fatty liver disease reflects the metabolic syndrome and visceral fat accumulation. Am J Gastroenterol 2007; 102(12):2708-15. doi: 10.1111/j.1572-0241.2007.01526.x [Crossref] [ Google Scholar]
- IPAQ Research Committee. Guidelines for the Data Processing and Analysis of the International Physical Activity Questionnaire (IPAQ)-Short and Long Forms. 2005. Available from: https://www.ipaq.ki.se/scoring. Accessed January 5, 2015.
- Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 1979; 95(2):351-8. doi: 10.1016/0003-2697(79)90738-3 [Crossref] [ Google Scholar]
- Angulo P, Hui JM, Marchesini G, Bugianesi E, George J, Farrell GC. The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology 2007; 45(4):846-54. doi: 10.1002/hep.21496 [Crossref] [ Google Scholar]
- Soleimani D, Rezaie M, Rajabzadeh F, Gholizadeh Navashenaq J, Abbaspour M, Miryan M. Protective effects of propolis on hepatic steatosis and fibrosis among patients with nonalcoholic fatty liver disease (NAFLD) evaluated by real-time two-dimensional shear wave elastography: a randomized clinical trial. Phytother Res 2021; 35(3):1669-79. doi: 10.1002/ptr.6937 [Crossref] [ Google Scholar]
- Salehi-Sahlabadi A, Chhabra M, Rahmani J, Momeni A, Karam G, Nattagh-Eshtivani E. The effect of propolis on anthropometric indices and lipid profile: a systematic review and meta-analysis of randomized controlled trials. J Diabetes Metab Disord 2020; 19(2):1835-43. doi: 10.1007/s40200-020-00604-2 [Crossref] [ Google Scholar]
- Koya-Miyata S, Arai N, Mizote A, Taniguchi Y, Ushio S, Iwaki K. Propolis prevents diet-induced hyperlipidemia and mitigates weight gain in diet-induced obesity in mice. Biol Pharm Bull 2009; 32(12):2022-8. doi: 10.1248/bpb.32.2022 [Crossref] [ Google Scholar]
- Gaweł S, Wardas M, Niedworok E, Wardas P. [Malondialdehyde (MDA) as a lipid peroxidation marker]. Wiad Lek 2004;57(9-10):453-5. [Polish].
- Yousef M, El-Nassag D, Gasser M, Ibrahim A. Potential protective effects of propolis against hepatotoxicity and nephrotoxicity induced by monosodium glutamate in rabbits. Alex Sci Exch J 2019; 40:30-42. doi: 10.21608/asejaiqjsae.2019.26639 [Crossref] [ Google Scholar]
- Nazari-Bonab H, Jamilian P, Radkhah N, Zarezadeh M, Ebrahimi-Mameghani M. The effect of propolis supplementation in improving antioxidant status: a systematic review and meta-analysis of controlled clinical trials. Phytother Res 2023; 37(9):3712-23. doi: 10.1002/ptr.7899 [Crossref] [ Google Scholar]
- Bankova V. Chemical diversity of propolis and the problem of standardization. J Ethnopharmacol 2005; 100(1-2):114-7. doi: 10.1016/j.jep.2005.05.004 [Crossref] [ Google Scholar]
- Calegari MA, Prasniewski A, da Silva C, Sado RY, Maia FM, Tonial LM. Propolis from Southwest of Parana produced by selected bees: Influence of seasonality and food supplementation on antioxidant activity and phenolic profile. An Acad Bras Cienc 2017; 89(1):45-55. doi: 10.1590/0001-3765201620160499 [Crossref] [ Google Scholar]
- Bonamigo T, Campos JF, Alfredo TM, Balestieri JB, Cardoso CA, Paredes-Gamero EJ. Antioxidant, cytotoxic, and toxic activities of propolis from two native bees in Brazil: Scaptotrigonadepilis and Melipona quadrifasciataanthidioides. Oxid Med Cell Longev 2017; 2017:1038153. doi: 10.1155/2017/1038153 [Crossref] [ Google Scholar]
- Cotelle N. Role of flavonoids in oxidative stress. Curr Top Med Chem 2001; 1(6):569-90. doi: 10.2174/1568026013394750 [Crossref] [ Google Scholar]
- Takashima M, Ichihara K, Hirata Y. Neuroprotective effects of Brazilian green propolis on oxytosis/ferroptosis in mouse hippocampal HT22 cells. Food Chem Toxicol 2019; 132:110669. doi: 10.1016/j.fct.2019.110669 [Crossref] [ Google Scholar]
- Itoh K, Tong KI, Yamamoto M. Molecular mechanism activating Nrf2-Keap1 pathway in regulation of adaptive response to electrophiles. Free Radic Biol Med 2004; 36(10):1208-13. doi: 10.1016/j.freeradbiomed.2004.02.075 [Crossref] [ Google Scholar]
- Zhang J, Shen X, Wang K, Cao X, Zhang C, Zheng H. Antioxidant activities and molecular mechanisms of the ethanol extracts of Baccharis propolis and Eucalyptus propolis in RAW64.7 cells. Pharm Biol 2016; 54(10):2220-35. doi: 10.3109/13880209.2016.1151444 [Crossref] [ Google Scholar]
- Silveira MA, Teles F, Berretta AA, Sanches TR, Rodrigues CE, Seguro AC. Effects of Brazilian green propolis on proteinuria and renal function in patients with chronic kidney disease: a randomized, double-blind, placebo-controlled trial. BMC Nephrol 2019; 20(1):140. doi: 10.1186/s12882-019-1337-7 [Crossref] [ Google Scholar]