Health Promotion Perspectives. 13(3):237-242.
doi: 10.34172/hpp.2023.29
Original Article
Diversity of Bacteroidaceae family in gut microbiota of patients with chronic kidney disease and end stage renal disease
Siamak Amini Khiabani Formal analysis, Investigation, Methodology, Visualization, Writing – original draft, Writing – review & editing, 1 
Setareh Haghighat Supervision, Writing – review & editing, 1 
Hamid Tayebi Khosroshahi Resources, Supervision, Writing – review & editing, 2 
Mohammad Asgharzadeh Supervision, Validation, Writing – review & editing, 3 
Hossein Samadi Kafil Conceptualization, Data curation, Formal analysis, Funding acquisition, Project administration, Resources, Supervision, Writing – original draft, Writing – review & editing, 4, * 
Author information:
1Department of Microbiology, Faculty of Advanced Science and Technology, Tehran Medical Sciences, Islamic Azad University, Tehran, Iran
2Research Center for Pharmaceutical Nanotechnology, Tabriz University of Medical Sciences, Tabriz, Iran
3Biotechnology Research Center, Tabriz University of Medical Sciences, Tabriz, Iran
4Drug Applied Research Center, Faculty of Medicine, Tabriz University of Medical Sciences, Tabriz, Iran
Abstract
Background:
Human intestine microbiota are known to be directly and indirectly altered during some diseases such as kidney complications. Bacteroides is considered as the main and the most abundant phylum among human gut microbiota, which has been classified as enterotype 1. This study aimed to assess the abundance of Bacteroides spp. in fecal flora of end-stage renal disease (ESRD) and chronic kidney disease (CKD) patients and compare it with the Bacteroides composition among fecal flora of healthy individual.
Methods:
Fresh fecal samples were collected from 20 CKD/ESRD patients and 20 healthy individual without any kidney complications. The pure microbial DNA was extracted by QIAamp Stool Mini Kit from stool samples. MiSeq system was used to analyze the intestinal composition by next generation sequencing method.
Results:
A number of 651 bacterial strains were isolated and identified from 40 fecal samples of both patients and healthy groups. Bioinformatics analysis defined 18 different types of Bacteroides species which included 2.76% of all strains. Statistical analysis showed no significant difference between study groups (P>0.05). In both healthy and patient groups three species including B. dorei, B. uniformis, and B. ovatus have allocated the most abundance to themselves. The lowest abundance was related to B. eggerthii, A. furcosa and B. barnesiae among CKD/ESRD patients and A. furcosa, B. barnesiae, and B. coprocola had the lowest abundance among healthy people.
Conclusion:
This study indicates despite all previous evidence of Bacteroides role in gut microbiota, it had no different distribution between healthy persons and CKD/ESRD patients.
Keywords: Bacteroidaceae, Chronic kidney disease, End-stage renal disease, Next generation sequencing
Copyright and License Information
© 2023 The Author(s).
This is an open access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Bacteroidaceae family is belonged to Bacteroidetes class and phylum. The phylum Bacteroidetes can be found in the environment for instance in sea water, soil, and sediments as well as colonized on the skin of animals and into the guts.1 Approximately, around 20%-80% of the gut microbiota relates to Bacteroidetes phylum in healthy adults which the genera of Parabacteroides, Bacteroides, Alistipes, and Prevotella are categorized in this phylum.2,3Bacteroidalesorder has a significant abundance in the human gut so that every gram of human feces has a high concentration, which reaches up to 109-1011 CFU.4 The genera Bacteroides has been identified as gram-negative, obligate anaerobes, rod-shaped with round ends, non-motile, and non-spore-forming which has known as one of the major genera of microbiota composition with more than 30 species.5,6
A large variety and myriad microorganisms have been colonized in human intestine which is called microbiota.7,8 Two major phyla: the gram-positive Firmicutes and the gram-negative high CG% Bacteroidetes have formed the microbiota population and the other phyla including Fusobacteria, Actinobacteria, and Verrucomicrobia phyla have been categorized at subdominant levels.5 Gut microbiota is a vast world that has many beneficial effects on human body, such as helping to food digestion, producing of hormones and essential vitamins like K and B12, modulating and developing of immune system and powerfully forming a natural defense to limit the infections caused by intestinal pathogens.9,10 Some studies also presented that the possible changes in species/phyla levels of Bacteroidetes and Firmicutes can be considered as obesity factors in children.11 Another study has reported that Bacteroides species could be widely effective in the treatment of intestinal colitis, metabolic disorders, immune dysfunctions, and cancer prevention which Bacteroides genus is considered as a new beneficial probiotic candidates.12 In addition, the members of Bacteroidaceae family also decreased inflammation response by regulating cytokine expression and lymphocytes.13
Chronic kidney disease (CKD) has known as a key determinant of noncommunicable disease which can progress toward end-stage renal disease (ESRD).14 Both developed and developing nations report high numbers of cases with CKD annually that can alter the intestinal microbiota composition and microbial metabolism quantitatively and qualitatively.15,16 Intestine microbiota has contributed to the production of important metabolic substances. On the other hand, the uremic solutes such as indoxyl sulfate and p-cresyl sulfate can be generated by gut microbiota.17 Patients who suffer from CKD have shown an altered combination of gut microbiota which have been correlated to the dietary interventions and therapeutic condition and the uremic milieu, result in high production of the uremic solutes.18 Several studies have reported a statistical association between mortality and circulating levels of the uremic toxins.18 In present study, we assessed changes in the abundance and diversity of Bacteroides spp. in intestinal flora of CKD and ESRD patients by comparing differences between healthy humans.
Materials and Methods
Sample collection
The present trial enrolled 20 patients with CKD and ESRD undergoing hemodialysis from the kidney transplantation ward of Imam-Reza teaching hospital, Tabriz, Iran. On the other hand, 20 healthy volunteers were joined to the study as control group. Fresh fecal samples of both case and control groups were directly collected from the anus of individuals and transferred into the sterile containers and were stored at -80 ºC until further processes. Before participating in the investigation, before participating in the research, written informed consent was signed by all patients and healthy group. Our ESRD patients in this investigation had some underlying diseases including chronic pyelonephritis, glomerulonephritis, hypertensive nephrosclerosis, polycystic kidney disease, post renal and urolithiasis, urolithiasis and systemic lupus erythematosus. Exclusion criteria include patients with some complications such as intestinal disease or colectomy, cholecystectomy, and diabetes, also patients suffering infections, inflammatory disorders, autoimmune diseases and patients who had received antibiotics within three months before enrolling in the study.
DNA extraction and PCR amplification
First of all four grams of mixed and homogenized fresh fecal samples were weighted for extraction of pure DNA. Microbial DNA was isolated from the fecal mixture using the QIAamp Stool Mini Kit (QIAGEN, Germany), according to the manufacturer’s instruction.19 The Thermo NanoDrop 2000 spectrophotometer (Thermo Scientific, MA, USA) was used to find the exact amount of DNA in each fecal sample.20 Two sequence of universal bacterial 16srRNA (V3–V4 hypervariable regions) were used for amplification of template DNA and sequencing. The specific sequences in this trial were as follows21:
Illumina V3:
5’-TCGTCGGCAGCGTCAGATGTGTATAA GAGACAGCCTACGGGNGGCWGCAG-3’
Illumina V4:
5’-TCTCGTGGGCTCGGAGATGTGTATAA GAGACAGGACTACHVGGGTATCTAATCC-3’
The amplification of the target sequences was performed using a T100TM thermal (Bio-Rad, USA). The polymerase chain reaction (PCR) reactions were performed as follows: 95 °C for 5 minutes, followed by 35 cycles of 95 °C for 1 minute, 55 °C for 45 seconds, and 72 °C for 1 minute, with a final extension of 72 °C for 1 minute. The electrophoresis was run in 1% agarose gel in Tris-boric acid-Ethylenediaminetetraacetic acid (EDTA) buffer to assess the PCR products and the gel was stained with ethidium-bromide to be visible under UV light. MiSeq system (100k 2 x 300 bp paired-end reads) (Illumina, USA) was accomplished the sequencing of PCR products in Omega Bioservices company. Bioinformatics analyses were completed by Illumina’s BaseSpace in parallel with Illumina’s in-house QIIme 2 pipeline.
Statistical analysis
Statistical analysis was assessed using programs including GraphPad PRISM 8 and SPSS 20. Statistical analysis was performed to compare case and control groups using the Mann-Whitney nonparametric test and Welch’s t test. P values < 0.05 were considered statistically significant.
Results
A total of 20 patients with CKD/ESRD, 14 patients were male and 6 patients were female with the mean age of 53.20 ± 12.03 years. As well as, a total of 20 healthy individuals, 10 persons were male and 10 were female with the mean age 59.3 ± 7.89 years. The results of MiSeq system demonstrated that 651 bacterial strains were found in 40 fecal samples of both patients and healthy individuals, which 18 strains (596538 reads, 257413 vs. 339125 reads) belong to family Bacteroidaceae. The strains were belonged to two genera including Bacteroides (17 species) and Anaerorhabdus (one species). The most abundance of species in patients with CKD/ESRD were B. dorei (32.66%), B. uniformis (21.03%) and B. ovatus (10.5%) and the lowest were B. eggerthii (0.01%), Anaerorhabdus furcosa (0.03%) and B. barnesiae (0.17%). As well as, the most abundance of species in healthy individuals were B. dorei (30.74%), B. uniformis (25.38%) and B. ovatus (18.3%) and the lowest were A. furcosa (0.01%), B. barnesiae (0.01%) and B. coprocola (0.07%). Using statistical analysis, the abundance of various species did not show any significant difference between the patients and control group (all P > 0.05). The abundance of various species is shown in Table 1 and Figure 1.
Table 1.
The abundance of different species of Bacteroidaceae family identified in fecal samples of both patients with CKD/ESRD and healthy individuals
|
Species
|
Patient group sum
|
Mean
|
STDEV
|
Min
|
Max
|
Individuals collected
|
Health individual sum
|
Mean
|
STDEV
|
Min
|
Max
|
Individuals collected
|
P
value
|
|
Bacteroides ovatus
|
26630 |
1331.5 |
3701.55 |
4 |
16463 |
20 |
62053 |
3102.65 |
8402.18 |
7 |
32760 |
20 |
0.396 |
|
Bacteroides dorei
|
84072 |
4203.6 |
8365.95 |
12 |
33205 |
20 |
104261 |
5213.05 |
12075.51 |
37 |
50687 |
20 |
0.760 |
|
Bacteroides uniformis
|
54126 |
2706.3 |
6549.13 |
2 |
28579 |
20 |
86058 |
4302.9 |
13938.53 |
0 |
62988 |
19 |
0.647 |
|
Bacteroides acidifaciens
|
554 |
27.7 |
116.65 |
0 |
523 |
4 |
1257 |
62.85 |
272.41 |
0 |
1220 |
5 |
0.600 |
|
Bacteroides coprocola
|
6411 |
320.55 |
1430.48 |
0 |
6398 |
4 |
241 |
12.05 |
36.83 |
0 |
142 |
3 |
0.347 |
|
Bacteroides caccae
|
13442 |
672.1 |
1214.01 |
0 |
3728 |
17 |
12198 |
609.9 |
1369.50 |
0 |
4739 |
15 |
0.880 |
|
Bacteroides thetaiotaomicron
|
6881 |
344.05 |
888.37 |
0 |
3823 |
13 |
8895 |
444.75 |
1516.11 |
0 |
6774 |
11 |
0.799 |
|
Bacteroides fragilis
|
14505 |
725.25 |
1919.34 |
0 |
8016 |
15 |
8267 |
413.35 |
1476.20 |
0 |
6643 |
13 |
0.568 |
|
Bacteroides massiliensis
|
4287 |
214.35 |
703.12 |
0 |
2948 |
10 |
4609 |
230.45 |
947.94 |
0 |
4253 |
9 |
0.952 |
|
Bacteroides faecis
|
11688 |
584.4 |
2612.57 |
0 |
11684 |
2 |
44 |
2.2 |
9.84 |
0 |
44 |
1 |
0.331 |
|
Bacteroides xylanisolvens
|
8919 |
445.95 |
1395.33 |
0 |
6314 |
15 |
11422 |
571.1 |
1606.37 |
0 |
6270 |
14 |
0.794 |
|
Bacteroides plebeius
|
18231 |
911.55 |
2889.86 |
0 |
11503 |
5 |
4331 |
216.55 |
653.14 |
0 |
2236 |
6 |
0.306 |
|
Bacteroides eggerthii
|
27 |
1.35 |
4.38 |
0 |
19 |
3 |
25678 |
1283.9 |
5712.37 |
0 |
25553 |
9 |
0.328 |
|
Bacteroides cellulosilyticus
|
3882 |
194.1 |
581.03 |
0 |
2460 |
9 |
5844 |
292.2 |
685.65 |
0 |
2394 |
14 |
0.628 |
|
Bacteroides clarus
|
2560 |
128 |
527.86 |
0 |
2369 |
10 |
2684 |
134.2 |
421.53 |
0 |
1848 |
10 |
0.967 |
|
Bacteroides nordii
|
672 |
33.6 |
87.98 |
0 |
371 |
4 |
704 |
35.2 |
107.60 |
0 |
458 |
6 |
0.959 |
|
Bacteroides barnesiae
|
442 |
22.1 |
97.90 |
0 |
438 |
2 |
540 |
27 |
120.75 |
0 |
540 |
1 |
0.889 |
|
Anaerorhabdus furcosa
|
84 |
4.2 |
14.81 |
0 |
66 |
3 |
39 |
1.95 |
8.72 |
0 |
39 |
1 |
0.562 |
STDEV, standard deviation; Min, minimum; Max, maximum.
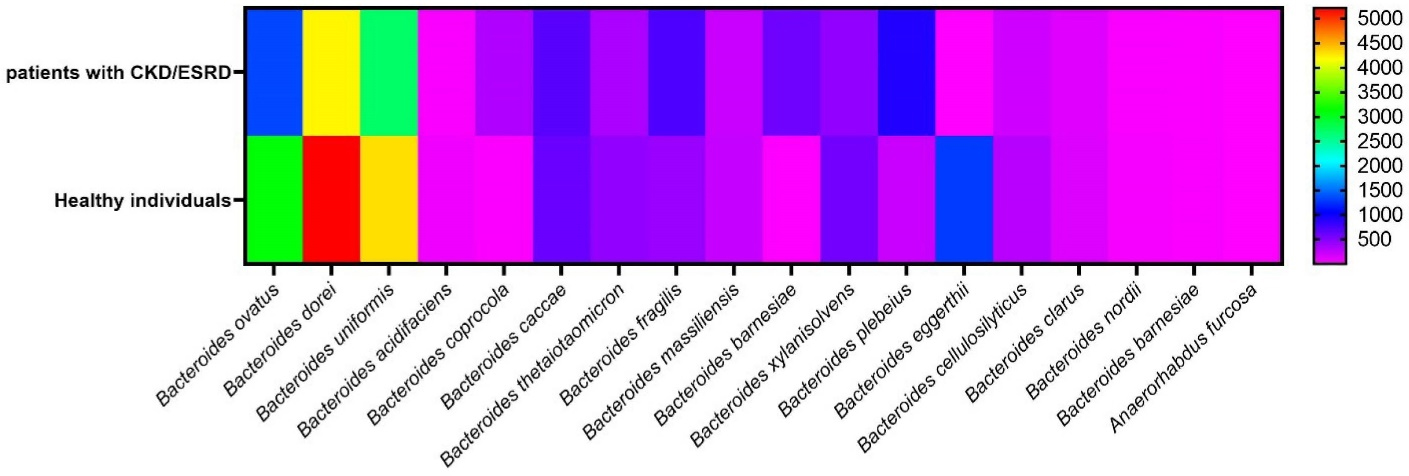
Figure 1.
Heatmap graph of the abundance of different species of Bacteroidaceae family identified in fecal samples of both patients with CKD/ESRD and healthy individuals. This map indicates number of each species of the family however statistical analysis showed no significant differences (P > 0.05)
.
Heatmap graph of the abundance of different species of Bacteroidaceae family identified in fecal samples of both patients with CKD/ESRD and healthy individuals. This map indicates number of each species of the family however statistical analysis showed no significant differences (P > 0.05)
Discussion
In both CKD patients and healthy individuals, Bacteroidetes (~40%), Firmicutes (~40%) and Proteobacteria (~10%) counted as the predominant phyla in gut microbiota composition.22 According to Faith et al23 report, during lifetime and human generations, Bacteroidetes phyla was more stable in comparison with the phyla Firmicutes. Other study has specified Bacteroidetes as the most plenteous phylum were accounted around 41% in both healthy clients and patients.24 In present study, among both CKD/ESRD patients and healthy volunteers, 18 strains of a total 651 bacterial strains were related to Bacteroidaceae family. On the other hand, the novelty of this study was to compare the abundance and diversity of Bacteroides species between fecal samples of CKD/ESRD patients and control group. Consequently, Statistical analysis calculations proved that there was no significant difference in variety of Bacteroides species between patients with CKD/ESRD and healthy individuals.
Numerous studies have examined Bacteroides at different levels of phylum, family, genus and species. Gut microbiota balance changed qualitatively and quantitatively through CKD patients who this imbalance is accompanied with decrease in Bacteroidaceae, some Prevotellaceae, and particular Bifidobacterium and Lactobacillus, and increase in the count of Enterobacteriaceae, Lachnospiraceae, and certain Ruminococcaceae.22,25 Jiang et al demonstrated that a significant reduction of total bacteria quantity was shown in ESRD patients. Bacteroides was prevalent in ESRD patients whereas Prevotella was enriched in healthy individuals. Also, among ESRD patients, the count of the butyrate producing bacteria such as Faecalibacterium, Roseburia, Coprococcus, Prevotella, and Clostridium were declined.22 Crespo-Salgado et al26 found that the gut microbiota composition in pediatric patients with hemodialysis was different compared with healthy controls. Unlike healthy individual, a decrease was shown in Proteobacteria members while Bacteroidetes was considerably increased in hemodialysis (HD) patients.26 In addition to phylum Bacteroidetes, this raises in HD patients, phylum Firmicutes decreases in ESRD patients undergoing peritoneal dialysis (PD).25
In other study about kidney stone disease (KSD), scientists found that gut microbiome can have an essential role in kidney stone formation.27 A unique gut microbiota was shown in patients who suffer from nephrolithiasis compared with healthy clients.27 Among kidney stone formers, Bacteroides spp. was more prevalent while healthy controls significantly had higher Prevotella spp. in microbiota composition.27 In a comparison of Bacteroides count among KSD and healthy group, Bacteroidetes was 3.4 times more plenty in KSD patients.27 Li et al assessed patients with both CKD and high systolic blood pressure and observed altered bacterial composition and a reduction in bacterial abundance. Their achievement demonstrated which in hypertension models, the richness of the intestinal Bacteroidetes and Firmicutes was associated with increased blood pressure.28
Total quantity of fecal microbiota was decreased in ESRD patients unlike healthy controls. Human intestinal microbiota has 3 main enterotype including Bacteroides categorized as enterotype 1, Prevotella as enterotype 2, and Ruminococcus as enterotype 3.29 A research study showed that from healthy individual through patients with ESRD, the mentioned enterotype shift from enterotype 2 (Prevotella) to enterotype 1 (Bacteroides), especially Bacteroidaceae which have the ability to produce p-cresol increase in ESRD patients.27 On the other hand, those bacteria that tend to produce short chain fatty acids like butyrate were declined among patients with ESRD.27
Several studies demonstrated that Bacteroides increase greatly in a variety of diseases, for instance Bacteroides in genus level has increased significantly in diabetes mellitus group.30 In addition, the segmented filamentous bacteria specially colonization by Bacteroidetes can induce the intestinal infiltration of pro-inflammatory TH17 cells, which is necessary to balance TH1 and TH2 responses.31
In present research, Bacteroides in genus and species level were assessed among both CKD/ESRD patients and healthy people which B. dorei, B. uniformis, and B. ovatus had the highest abundant among both group without any difference. Boente et al32 have evaluated the members of Bacteroidaceae among other disease like hypertension that some species of Bacteroides including B. eggerthii, B. cellulosilyticus, and 3 unclassified Bacteroides had important function in patient with hypertension and other Bacteroides spp. such as B. dorei, B. nordii, and B. uniformis have enriched in control group. These findings refer to that the composition of Bacteroidaceae members alter not only among CKD/ESRD patients, but also in some other disorders.
Conclusion
In summary, several previous knowledge have demonstrated the correlation between increased abundance of Bacteroides members and some disease particularly Kidney problems. Therefore, our findings around comparison of Bacteroides species abundance with CKD/ESRD patients and healthy individuals can extend the previous findings, which there was no significant difference in distribution of Bacteroidaceaemembers among both assessed patients and healthy groups.
The limitation of our research is that due to financial limitations, it was not possible to conduct research on a larger number of individuals.
Acknowledgements
We thank all support for colleagues specially Dr. Pourya Gholizadeh for helps in manuscript preparation and comments.
Competing Interests
None to declare.
Ethical Approval
This study was conducted based on the confirmation of Medical Ethics Board of Trustees with reference number IR.IAU.PS.REC.1400.483. The result of the Medical Ethics Board of Trustees report is available online (https://ethics.research.ac.ir/EthicsProposalViewEn.php?id = 247843)
Funding
This study was done as a dissertation for the Ph.D. degree of Dr. Siamak Amini Khabani and was supported by self-grant and Tabriz University of Medical Sciences and Islamic Azad University. This study was approved in Islamic Azad University with reference number 162314446.
References
- O’Connor K, Morrissette M, Strandwitz P, Ghiglieri M, Caboni M, Liu H. Cranberry extracts promote growth of Bacteroidaceae and decrease abundance of Enterobacteriaceae in a human gut simulator model. PLoS One 2019; 14(11):e0224836. doi: 10.1371/journal.pone.0224836 [Crossref] [ Google Scholar]
- Huttenhower C, Gevers D, Knight R, Abubucker S, Badger JH, Chinwalla AT. Structure, function and diversity of the healthy human microbiome. Nature 2012; 486(7402):207-14. doi: 10.1038/nature11234 [Crossref] [ Google Scholar]
- Gholizadeh P, Pormohammad A, Eslami H, Shokouhi B, Fakhrzadeh V, Samadi Kafil H. Oral pathogenesis of Aggregatibacter actinomycetemcomitans. Microb Pathog 2017; 113:303-11. doi: 10.1016/j.micpath.2017.11.001 [Crossref] [ Google Scholar]
- Zitomersky NL, Atkinson BJ, Franklin SW, Mitchell PD, Snapper SB, Comstock LE. Characterization of adherent bacteroidales from intestinal biopsies of children and young adults with inflammatory bowel disease. PLoS One 2013; 8(6):e63686. doi: 10.1371/journal.pone.0063686 [Crossref] [ Google Scholar]
- Eckburg PB, Bik EM, Bernstein CN, Purdom E, Dethlefsen L, Sargent M. Diversity of the human intestinal microbial flora. Science 2005; 308(5728):1635-8. doi: 10.1126/science.1110591 [Crossref] [ Google Scholar]
- Lay C, Doré J, Rigottier-Gois L. Separation of bacteria of the Clostridium leptum subgroup from the human colonic microbiota by fluorescence-activated cell sorting or group-specific PCR using 16S rRNA gene oligonucleotides. FEMS Microbiol Ecol 2007; 60(3):513-20. doi: 10.1111/j.1574-6941.2007.00312.x [Crossref] [ Google Scholar]
- Gholizadeh P, Mahallei M, Pormohammad A, Varshochi M, Ganbarov K, Zeinalzadeh E. Microbial balance in the intestinal microbiota and its association with diabetes, obesity and allergic disease. Microb Pathog 2019; 127:48-55. doi: 10.1016/j.micpath.2018.11.031 [Crossref] [ Google Scholar]
- Ebrahimzadeh Leylabadlo H, Ghotaslou R, Feizabadi MM, Farajnia S, Moaddab SY, Ganbarov K. The critical role of Faecalibacterium prausnitzii in human health: an overview. Microb Pathog 2020; 149:104344. doi: 10.1016/j.micpath.2020.104344 [Crossref] [ Google Scholar]
- Sekirov I, Russell SL, Antunes LC, Finlay BB. Gut microbiota in health and disease. Physiol Rev 2010; 90(3):859-904. doi: 10.1152/physrev.00045.2009 [Crossref] [ Google Scholar]
- Homayouni Rad A, Aghebati Maleki L, Samadi Kafil H, Fathi Zavoshti H, Abbasi A. Postbiotics as novel health-promoting ingredients in functional foods. Health Promot Perspect 2020; 10(1):3-4. doi: 10.15171/hpp.2020.02 [Crossref] [ Google Scholar]
- dos Santos Pereira Indiani CM, Rizzardi KF, Castelo PM, Ferraz LFC, Darrieux M, Parisotto TM. Childhood obesity and Firmicutes/Bacteroidetes ratio in the gut microbiota: a systematic review. Child Obes 2018; 14(8):501-9. doi: 10.1089/chi.2018.0040 [Crossref] [ Google Scholar]
- Sanders ME, Shane AL, Merenstein DJ. Advancing probiotic research in humans in the United States: challenges and strategies. Gut Microbes 2016; 7(2):97-100. doi: 10.1080/19490976.2016.1138198 [Crossref] [ Google Scholar]
- Tan H, Zhai Q, Chen W. Investigations of Bacteroides spp. towards next-generation probiotics. Food Res Int 2019; 116:637-44. doi: 10.1016/j.foodres.2018.08.088 [Crossref] [ Google Scholar]
- Couser WG, Remuzzi G, Mendis S, Tonelli M. The contribution of chronic kidney disease to the global burden of major noncommunicable diseases. Kidney Int 2011; 80(12):1258-70. doi: 10.1038/ki.2011.368 [Crossref] [ Google Scholar]
- Hida M, Aiba Y, Sawamura S, Suzuki N, Satoh T, Koga Y. Inhibition of the accumulation of uremic toxins in the blood and their precursors in the feces after oral administration of Lebenin, a lactic acid bacteria preparation, to uremic patients undergoing hemodialysis. Nephron 1996; 74(2):349-55. doi: 10.1159/000189334 [Crossref] [ Google Scholar]
- Laffin MR, Tayebi Khosroshahi H, Park H, Laffin LJ, Madsen K, Samadi Kafil H. Amylose resistant starch (HAM-RS2) supplementation increases the proportion of Faecalibacterium bacteria in end-stage renal disease patients: microbial analysis from a randomized placebo-controlled trial. Hemodial Int 2019; 23(3):343-7. doi: 10.1111/hdi.12753 [Crossref] [ Google Scholar]
- Yacoub R, Wyatt CM. Manipulating the gut microbiome to decrease uremic toxins. Kidney Int 2017; 91(3):521-3. doi: 10.1016/j.kint.2017.01.003 [Crossref] [ Google Scholar]
- Lin CJ, Wu V, Wu PC, Wu CJ. Meta-analysis of the associations of p-Cresyl sulfate (PCS) and indoxyl sulfate (IS) with cardiovascular events and all-cause mortality in patients with chronic renal failure. PLoS One 2015; 10(7):e0132589. doi: 10.1371/journal.pone.0132589 [Crossref] [ Google Scholar]
- Johnson CA, Levey AS, Coresh J, Levin A, Lau J, Eknoyan G. Clinical practice guidelines for chronic kidney disease in adults: part I. Definition, disease stages, evaluation, treatment, and risk factors. Am Fam Physician 2004; 70(5):869-76. [ Google Scholar]
- Samadi Kafil H, Mohabati Mobarez A. Assessment of biofilm formation by enterococci isolates from urinary tract infections with different virulence profiles. J King Saud Univ Sci 2015; 27(4):312-7. doi: 10.1016/j.jksus.2014.12.007 [Crossref] [ Google Scholar]
- Klindworth A, Pruesse E, Schweer T, Peplies J, Quast C, Horn M. Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucleic Acids Res 2013; 41(1):e1. doi: 10.1093/nar/gks808 [Crossref] [ Google Scholar]
- Jiang S, Xie S, Lv D, Wang P, He H, Zhang T. Alteration of the gut microbiota in Chinese population with chronic kidney disease. Sci Rep 2017; 7(1):2870. doi: 10.1038/s41598-017-02989-2 [Crossref] [ Google Scholar]
- Faith JJ, Guruge JL, Charbonneau M, Subramanian S, Seedorf H, Goodman AL. The long-term stability of the human gut microbiota. Science 2013; 341(6141):1237439. doi: 10.1126/science.1237439 [Crossref] [ Google Scholar]
- Cigarran Guldris S, González Parra E, Cases Amenós A. Gut microbiota in chronic kidney disease. Nefrología (Engl Ed) 2017; 37(1):9-19. doi: 10.1016/j.nefroe.2017.01.017 [Crossref] [ Google Scholar]
- Amini Khiabani S, Haghighat S, Tayebi Khosroshahi H, Asgharzadeh M, Samadi Kafil H. Clostridium species diversity in gut microbiota of patients with renal failure. Microb Pathog 2022; 169:105667. doi: 10.1016/j.micpath.2022.105667 [Crossref] [ Google Scholar]
- Crespo-Salgado J, Vehaskari VM, Stewart T, Ferris M, Zhang Q, Wang G. Intestinal microbiota in pediatric patients with end stage renal disease: a Midwest Pediatric Nephrology Consortium study. Microbiome 2016; 4(1):50. doi: 10.1186/s40168-016-0195-9 [Crossref] [ Google Scholar]
- Stern JM, Moazami S, Qiu Y, Kurland I, Chen Z, Agalliu I. Evidence for a distinct gut microbiome in kidney stone formers compared to non-stone formers. Urolithiasis 2016; 44(5):399-407. doi: 10.1007/s00240-016-0882-9 [Crossref] [ Google Scholar]
- Li J, Zhao F, Wang Y, Chen J, Tao J, Tian G. Gut microbiota dysbiosis contributes to the development of hypertension. Microbiome 2017; 5(1):14. doi: 10.1186/s40168-016-0222-x [Crossref] [ Google Scholar]
- Arumugam M, Raes J, Pelletier E, Le Paslier D, Yamada T, Mende DR. Enterotypes of the human gut microbiome. Nature 2011; 473(7346):174-80. doi: 10.1038/nature09944 [Crossref] [ Google Scholar]
- Tao S, Li L, Li L, Liu Y, Ren Q, Shi M. Understanding the gut-kidney axis among biopsy-proven diabetic nephropathy, type 2 diabetes mellitus and healthy controls: an analysis of the gut microbiota composition. Acta Diabetol 2019; 56(5):581-92. doi: 10.1007/s00592-019-01316-7 [Crossref] [ Google Scholar]
- Yang T, Richards EM, Pepine CJ, Raizada MK. The gut microbiota and the brain-gut-kidney axis in hypertension and chronic kidney disease. Nat Rev Nephrol 2018; 14(7):442-56. doi: 10.1038/s41581-018-0018-2 [Crossref] [ Google Scholar]
- Yan Q, Gu Y, Li X, Yang W, Jia L, Chen C. Alterations of the gut microbiome in hypertension. Front Cell Infect Microbiol 2017; 7:381. doi: 10.3389/fcimb.2017.00381 [Crossref] [ Google Scholar]