The effects of acute exercise intensity on episodic and false memory among young adult college students
Health Promotion Perspectives, 9(2), 143-149; DOI:10.15171/hpp.2019.20
Original Article
The effects of acute exercise intensity on episodic and false memory among young adult college students
Emma K. Dilley1, Liye Zou2, Paul D. Loprinzi1 ,*
1
Exercise & Memory Laboratory, Department of Health, Exercise Science and Recreation Management, The University of Mississippi, University, MS 38677, USA
2
Lifestyle (Mind-Body Movement) Research Center, College of Sport Science, Shenzhen University, Shenzhen 518060, China
Email: pdloprin@olemiss.edu
© 2019 The Author(s). This is an open access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Background: Previous experimental work demonstrates that acute exercise may enhance episodic memory performance. However, limited research has examined the extent to which acute exercise influences false episodic memory production, and no studies, to date, have examined whether there is an intensity-specific effect of acute exercise on both true episodic and false episodic memories. Thus, the present experiment evaluated the effects of intensity-specific acute exercise on episodic memory and false episodic memory.
Methods: A three-arm, parallel, between-group randomized controlled trial was employed in the University setting, with participants (N=60; Mage= 20.8 years) randomized into a moderate intensity exercise group (15-minute bout of treadmill exercise at 50% heart rate reserve), a high intensity exercise group (15-minute bout of treadmill exercise at 80% heart rate reserve), or a control group (time-matched period of sitting). True episodic and false episodic memory were both assessed using 6 word-lists from the Deese-Roediger-McDermott (DRM) paradigm, including both a short-term recall and a delayed memory recognition assessment.
Results: For the number of words recalled across each of the 6 lists, there was a significant main effect for list (P<0.001, η2p=0.15), marginally significant main effect for group (P=0.07, η2p=0.09), but no list by group interaction effect (P=0.44, η2p=0.03). Those in the high-intensity exercise group recalled significantly (P<0.05) more words than the control group. For the false episodic word recall, across various lists, high-intensity acute exercise was associated with a greater rate of false episodic memories. For the memory recognition task, there was no main effect for word type (P=0.46, η2p=0.01), group (P=0.4443, η2p=.03), word type by group interaction (P=0.44,η2p=0.03), recall by group interaction (P=0.4441, η2p=0.04), or word type by recall by group interaction (P=0.32, η2p=0.04). However, there was a main effect for recall (P<0.001, η2p=.54)and a word type by recall interaction (P<0.001, η2p=0.77).Conclusion: These findings suggest that acute high-intensity exercise may enhance true episodic memories, and, possibly, also increase the rate of false episodic memories. We discuss these findings in the context of how different acute exercise intensities may have unique and differential effects on underlying mechanistic processes related to true and false episodic memory.
Keywords: Aerobic exercise, Cognition, Fuzzy trace theory, Hippocampus, Memory distortion, Prefrontal cortex, Recognition, Recollection, Short-term memory
Citation: Dilley EK, Zou L, Loprinzi PD. The effects of acute exercise intensity on episodic and false memory among young adult college students. Health Promot Perspect. 2019;9(2):143-149. doi: 10.15171/hpp.2019.20.
Introduction
Emerging research demonstrates that acute exercise can help to facilitate episodic retrospective memory,1 but to date, few studies have examined the effects of exercise on false episodic memory function. False episodic memories can be conceptualized as a fabricated or distorted recollection of an event. Previous research has discussed potential mechanisms of false episodic memory.2-5 In brief, false episodic memories, or memory distortions, may arise from culturally determined expectations, labeling of the memory/event, and imperfect reality monitoring processes, such as source monitoring, which includes attributions about the origin of activated information. The source monitoring framework6 is perhaps one of the more extensive theoretical accounts of false episodic memories, which highlights several key aspects of false episodic memories.
These key aspects indicate that memory attributions arise from 1) various qualitative characteristics of the mental experience (e.g., perceptual, spatial, temporal, or emotional details), 2) the embeddedness of the mental experience (e.g., availability of supporting memories), and 3) goals, beliefs, motivation, and social factors. Per this model, false episodic memories occur because mental experiences arising from different events have overlapping characteristics that are imperfectly differentiated. Additional work also indicates that episodic memory and executive function performance predicts false episodic memory function.4 Both of these cognitive functions have been shown to be influenced by acute exercise,7,8 providing plausibility for a potential relationship between acute exercise and false episodic memory performance. Further, acute exercise may subserve the encoding of contextually specific information (reactivate verbatim memory traces and attenuate the reactivation of gist traces9), and in turn, minimize false episodic memory recall.
Lastly, as we have recently discussed,10 few studies have examined the potential intensity-specific effects of acute exercise on episodic memory, let alone false episodic memory function. Intensity-specific effects of acute exercise on memory are plausible; as discussed elsewhere,10 higher-intensity exercise may more favorably subserve long-term potentiation,11 and in turn, episodic memory performance. To address these two gaps in the literature, the purpose of this experiment was to evaluate the effects of acute exercise, across varying intensities, on true episodic memory and false episodic memory performance.
Materials and Methods
Study design
This experiment was approved by the authors’ institutional review board and participants provided written consent prior to participation. The present study was a three-arm, between-group randomized controlled trial, consisting of two exercise experimental groups and a control group. The senior author (PL) generated the randomization via a computer-facilitated algorithm. The lead researcher (ED) enrolled the participants and maintained allocation concealment by waiting to assign them to the group until after recruitment and consent was obtained. The exercise groups engaged in an acute 15-minute bout of moderate-intensity exercise or high-intensity exercise. The control group completed a time-matched seated task (on-line game). Both groups completed one laboratory visit in the authors’ Exercise & Memory Laboratory. Data collection occurred from September to December of 2018. Primary outcomes for this experiment included the true episodic memory and false episodic memory measures (described below). Secondary outcomes included physiological (e.g., heart rate) and psychological (e.g., ratings of perceived exercise) responses from the acute bout of exercise.
Participants and procedures
All three groups included 20 participants (N=60). This is based from a power analysis indicating a sample size of 20 would be needed for sufficient power (d, 0.90; two-tailed α error probability, 0.05; 1-β error probability, 0.80). This was informed from other related work.1,12,13 We recruited through classroom announcements and word-of-mouth. Participants included male and females between the ages of 18 to 35 years. Additionally, participants were excluded if they: 1) Self-reported as a daily smoker14,15; 2) Self-reported being pregnant16; 3) Exercised within 5 hours of testing1; 4) Consumed caffeine within 3 hours of testing17; 5) Took medications used to regulate emotion (e.g., SSRI’s)18; 6) Had a concussion or head trauma within the past 30 days19; 7) Took marijuana or other illegal drugs within the past 30 days20; or 8) Were considered a daily alcohol user (> 30 drinks/month for women; > 60 drinks/month for men).21
Experimental conditions
Similar to other related research,22 the control condition played a medium-level, on-line administered, Sudoku puzzle. Participants in this control group completed this time-matched puzzle for 20-minutes prior to completing the memory task (described below). The website for this puzzle is located here: https://www.websudoku.com/.
The two exercise conditions (moderate-intensity and vigorous-intensity) engaged in a 15-minute bout of treadmill exercise, followed by a 5-min recovery period. The HRR equation used to evaluate exercise intensity is:
HRR = [(HRmax - HRrest) * % intensity] + HRrest
To calculate HRrest, at the beginning of the visit, participants sat quietly for 5 minutes, and HR was recorded from a Polar HR monitor. HRmax was estimated from the 220-age formula. For the moderate-intensity and vigorous-intensity exercise, respectively, 50% and 80% will be entered into the above formula. These respective intensities represent moderate- and vigorous-intensity exercise.23
Memory assessment
The procedure for this false episodic memory task was modeled after Roediger and McDermott.24 Participants listened (via headphones) to a recording of a list of 15 words; each word was read at a rate of 1 word per 1.5 seconds. They listened to six separate word lists. After each list, they were asked to write down (on paper) all the words they could remember from the list. As an example, each list was composed of associates (e.g., bed, rest, awake) of 1 non-presented word/lure (e.g., sleep). If, for example, they wrote down the word “sleep”, then this was evidence of constructing a false episodic memory. The lure word for list 1 was “mountain” and example studied words for this list were: hill, valley, and climb. The lure word for list 2 was “needle” and example studied words for this list were: thread, pin, eye. The lure word for list 3 was “chair” and example studied words for this list were: table, sit, and legs. The lure word for list 4 was “rough” and example studied words for this list were: smooth, bumpy, and road. The lure word for list 5 was “sleep” and example studied words for this list were: bed, rest, and awake. The lure word for list 6 was “sweet” and example studied words for this list were: sour, candy, and sugar.
After their recall of the 6th list, participants watched an on-line video (The Office Bloopers) for 10-minutes as a distractor task. After this, we assessed their false episodic memory recognition by giving them a piece of paper that has 42 words on it. Of these 42 words, 12 were words that they studied from one of the previous 6 lists. However, 30 were non-studied words. Among the 30 non-studied words, 6 were critical words/lures from which the lists were generated (e.g., sleep), 12 were unrelated to any of the items on the list, and 12 were related to the words on the lists (2 per list). The 42 items were subdivided into 6 blocks, with each block consisting of 7 items. Each block included 2 studied words, 2 related words, 2 unrelated words and the critical non-studied word/lure. For each of the 42 items, they were asked to rate the item on a 4-point scale, including the following response options: 4 for sure that they item was old (studied); 3 for probably old, 2 for probably new, and 1 for sure it was new.
Statistical analysis
All statistical analyses were computed in JASP (v. 0.9.1). The proportion of items classified as Sure Old (a rating of 4), Probably Old (3), Probably New (2) and Sure New (1) were calculated. A 3 (group) x 4 (memory recognition categories) x 4 (word type; studied, unrelated lure, weakly related lure, or a critical lure) ANOVA was employed for the false episodic memory recognition assessment. For the number of words recalled, a 3 (group) x 6 (number of word lists) ANOVA was employed. Statistical significance was set at a nominal alpha of 0.05. Post hoc t tests were employed when main and interaction effects were statistically significant or approached statistical significance. Notably, we employed post-hoc tests when main or interaction effects also approached significance because relying on a specific threshold (e.g., 0.05) for statistical significance is problematic.25,26 Partial eta-squared (η2p) was calculated for effect size estimates.
Results
Table 1 displays the demographic and behavioral characteristics of the sample. There were no significant differences in these parameters across the experimental groups. We also had no losses or exclusions after randomization.
|
Table 1. Demographic and behavioral characteristics of the sample
|
|
Variable
|
Control (n = 20)
|
Moderate-Intensity (n
= 20)
|
High-Intensity (
N
= 20)
|
P
value
|
| Age, mean years |
20.5 (1.1) |
20.8 (1.1) |
21.1 (0.9) |
0.31 |
| Gender, % female |
80.0 |
95.0 |
95.0 |
0.19 |
| Race-Ethnicity, % White |
70.0 |
70.0 |
80.0 |
0.88 |
| BMI, mean kg/m2 |
27.5 (6.2) |
25.0 (5.8) |
25.7 (6.2) |
0.42 |
| MVPA, mean min/wk |
131.9 (116.1) |
103.3 (91.1) |
153.4 (106.5) |
0.33 |
| Affect, mean |
|
|
|
|
| Positive |
28.2 (7.0) |
29.0 (7.2) |
27.5 (7.9) |
0.80 |
| Negative |
12.3 (2.4) |
13.4 (3.6) |
13.0 (2.7) |
0.50 |
|
BMI, body mass index; MVPA, moderate-to-vigorous physical activity.
Values in parentheses are standard deviations; P value is calculated from a one-way ANOVA (continuous variables) or chi-square analysis (categorical variables).
|
Table 2 displays the physiological (heart rate) and psychological (rating of perceived exertion, RPE) responses to the experimental conditions. For both heart rate (F(6,171)=132.9, P < 0.001, η2p= 0.82) and RPE (F(6,171)=81.7, P < 0.001, η2p= 0.74), there was a significant time by group interaction. In the control group, heart rate remained in the upper 70’s and low 80’s bpm; in the moderate-intensity exercise group, heart rate increased from 81.7 bpm to 140 bpm (P < 0.001); and in the acute high-intensity exercise group, heart rate increased from 77.6 bpm to 170.2 bpm (P < 0.001).
|
Table 2. Physiological (heart rate) and psychological (rating of perceived exertion) responses
|
|
Variable
|
Control (n
= 20)
|
Moderate-Intensity (n
= 20)
|
High-Intensity (n
= 20)
|
P
value
|
| Heart rate, mean bpm |
|
|
|
|
| Rest |
79.9 (12.6) |
81.7 (11.7) |
77.6 (18.3) |
F(3,171; time)=466.9, P < 0.001, η2p=0.89
F(2,57; group)=81.6, P < 0.001, η2p=0.74
F(6,171; time x group)=132.9, P < 0.001, η2p=0.82
|
| Midpoint |
80.8 (11.7) |
138.8 (12.7) |
149.9 (19.6) |
|
| Endpoint |
79.8 (11.2) |
140.1 (7.2) |
170.2 (13.1) |
|
| 5-Minutes post |
82.2 (10.9) |
91.2 (13.8) |
97.0 (14.3) |
|
| RPE, mean |
|
|
|
|
| Rest |
6.3 (0.9) |
6.0 (0.0) |
6.0 (0.0) |
F(3,171; time)=320.9, P < 0.001, η2p=0.85
F(2,57; group)=35.4, P < 0.001, η2p=0.55
F(6,171; time x group)=81.7, P < 0.001, η2p=0.74
|
| Midpoint |
6.6 (1.1) |
11.1 (1.4) |
11.4 (1.7) |
|
| Endpoint |
6.9 (2.4) |
11.4 (1.3) |
14.3 (1.6) |
|
| 5-Minutes post |
6.9 (2.2) |
6.5 (0.8) |
7.1 (1.1) |
|
Table 3 and Figure 1 display the episodic memory recall scores across the experimental groups. For the number of words recalled across each list, there was a significant main effect for list (F(5,285)=10.2, P < 0.001, η2p= 0.15), marginally significant main effect for group (F(2,57)=2.7, P = 0.07, η2p= 0.09), but no list by group interaction effect (F(10,285)=1.00, P = 0.44, η2p= 0.03). Across the 6 lists, those in the high-intensity exercise group recalled significantly (P < 0.05) more words than the control group for List 1, List 2, and List 5. Similarly, the moderate-intensity exercise group recalled significantly more words than the control group for List 2.
|
Table 3. True and false episodic memory recall scores across the three groups
|
|
Variable
|
Control (n
= 20)
|
Moderate-Intensity (n
= 20)
|
High-Intensity (n
= 20)
|
P
value
|
|
True episodic word recall
|
|
|
|
F(5,285; list)=10.2, P<0.001, η2p=0.15
F(2,57; group)=2.7, P=0.07, η2p=0.09
F(10,285; list x group)=1.00, P=0.44, η2p=0.03 |
| List 1, mean # words |
6.4 (1.2) |
6.8 (1.4) |
7.4 (1.7)a |
|
| List 2, mean # words |
6.7 (1.2) |
6.9 (1.7)c |
8.2 (1.6)b |
|
| List 3, mean # words |
8.0 (1.9) |
8.2 (2.7) |
8.5 (1.6) |
|
| List 4, mean # words |
6.4 (2.0) |
7.3 (2.1) |
6.9 (1.4) |
|
| List 5, mean # words |
7.0 (1.5) |
7.8 (2.2) |
8.6 (1.7)b |
|
| List 6, mean # words |
7.8 (2.0) |
7.9 (1.7) |
8.5 (2.2) |
|
|
False episodic word recall
|
|
|
|
F(5,285; list)=2.15, P=0.06, η2p=0.04
F(2,57; group)=2.20, P=0.12, η2p=0.07
F(10,285; list x group)=1.27, P=0.24, η2p=.04 |
| List 1, % false recall |
35.0 (48.9) |
30.0 (47.0) |
25.0 (44.4) |
|
| List 2, % false recall |
50.0 (51.3) |
55.0 (51.0) |
60.0 (50.3) |
|
| List 3, % false recall |
30.0 (47.0) |
50.0 (51.3) |
50.0 (51.3) |
|
| List 4, % false recall |
35.0 (48.9) |
45.0 (51.0) |
70.0 (47.0)a |
|
| List 5, % false recall |
45.0 (51.0) |
45.0 (51.0) |
50.0 (51.3) |
|
| List 6, % false recall |
20.0 (41.0) |
60.0 (50.3)c |
65.0 (48.9)b |
|
|
a High-intensity different (P < 0.05) than control.
b High-intensity different (P < 0.01) than control.
c Moderate-intensity different (P < 0.05) than control.
|
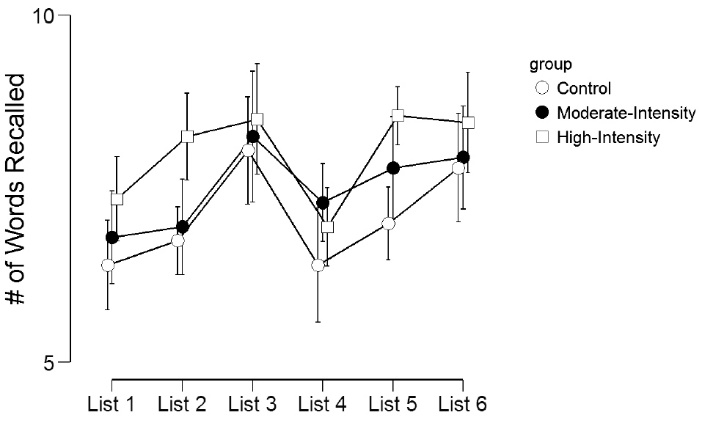
Figure 1. Number of words recalled across the 6 memory lists.
The proportion of false episodic word recall is shown in Table 3 and Figure 2. There was no significant main effect for list (F(5,285)=2.15, P = 0.06, η2p= 0.04), group (F(2,57)=2.20, P = 0.12, η2p= 0.07) or list by group interaction (F(10,285)=1.27, P = 0.24, η2p= 0.04).
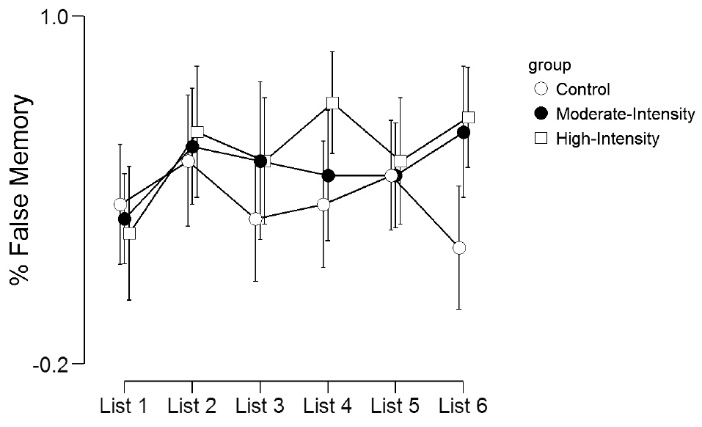
Figure 2. Proportion of false memories across the 6 lists.
Table 4 and Figure 3 display the memory recognition results. Word type refers to whether it was a studied, unrelated lure, weakly related lure, or a critical lure. Recall type refers to whether it was responded as sure old, probably old, probably new, or sure new. There was no main effect for word type (F(3,495) =0.85, P = 0.46, η2p= 0.01), group (F(2,55)=0.85, P = 0.43, η2p= 0.03), word type by group interaction (F(6,495)=0.97, P = 0.44, η2p= 0.03), recall by group interaction (F(6,495)=1.03, P = 0.41, η2p= 0.04), or word type by recall by group interaction (F(9,495)=1.13, P = 0.32, η2p= 0.04). However, there was a main effect for recall (F(3,495)=64.3, P < 0.001, η2p= 0.54) and a word type by recall interaction (F(9,495)=182.6, P < 0.001, η2p= 0.77). That is, participants across all three experimental conditions were more likely to perceive the studied and critical lure words as being “old” (i.e., that they previously were exposed to them during the study session).
|
Table 4. Memory recognition scores for each word type across the three groups (N=60)
|
|
Word Type
|
Recall
|
Group
|
Mean
|
SD
|
| Studied |
Sure Old |
Control |
69.456 |
15.403 |
|
|
Moderate-Intensity |
77.920 |
14.113 |
|
|
High-Intensity |
76.700 |
12.267 |
|
Probably Old |
Control |
12.956 |
11.163 |
|
|
Moderate-Intensity |
12.915 |
11.930 |
|
|
High-Intensity |
10.820 |
9.405 |
|
Probably New |
Control |
9.261 |
12.086 |
|
|
Moderate-Intensity |
5.835 |
7.215 |
|
|
High-Intensity |
6.735 |
5.027 |
|
Sure New |
Control |
8.328 |
7.571 |
|
|
Moderate-Intensity |
3.330 |
5.673 |
|
|
High-Intensity |
4.995 |
8.284 |
| Unrelated Lure |
Sure Old |
Control |
3.239 |
5.819 |
|
|
Moderate-Intensity |
2.495 |
4.757 |
|
|
High-Intensity |
1.665 |
3.417 |
|
Probably Old |
Control |
5.089 |
6.485 |
|
|
Moderate-Intensity |
7.920 |
10.289 |
|
|
High-Intensity |
8.750 |
9.928 |
|
Probably New |
Control |
34.722 |
26.546 |
|
|
Moderate-Intensity |
35.410 |
17.502 |
|
|
High-Intensity |
37.085 |
26.423 |
|
Sure New |
Control |
56.944 |
26.555 |
|
|
Moderate-Intensity |
54.170 |
21.552 |
|
|
High-Intensity |
52.500 |
29.878 |
| Weakly Related Lure |
Sure Old |
Control |
19.917 |
10.366 |
|
|
Moderate-Intensity |
17.905 |
10.905 |
|
|
High-Intensity |
17.075 |
9.922 |
|
Probably Old |
Control |
14.806 |
13.266 |
|
|
Moderate-Intensity |
20.430 |
8.334 |
|
|
High-Intensity |
23.330 |
14.700 |
|
Probably New |
Control |
26.394 |
21.252 |
|
|
Moderate-Intensity |
38.715 |
18.635 |
|
|
High-Intensity |
30.420 |
16.933 |
|
Sure New |
Control |
38.883 |
27.421 |
|
|
Moderate-Intensity |
22.920 |
18.109 |
|
|
High-Intensity |
29.170 |
22.541 |
| Critical Lure |
Sure Old |
Control |
70.361 |
25.276 |
|
|
Moderate-Intensity |
81.650 |
19.426 |
|
|
High-Intensity |
74.985 |
19.870 |
|
Probably Old |
Control |
14.778 |
17.957 |
|
|
Moderate-Intensity |
10.835 |
15.550 |
|
|
High-Intensity |
12.510 |
16.110 |
|
Probably New |
Control |
5.556 |
11.428 |
|
|
Moderate-Intensity |
6.675 |
9.977 |
|
|
High-Intensity |
5.835 |
11.177 |
|
Sure New |
Control |
9.267 |
14.262 |
|
|
Moderate-Intensity |
0.835 |
3.734 |
|
|
High-Intensity |
6.670 |
11.341 |
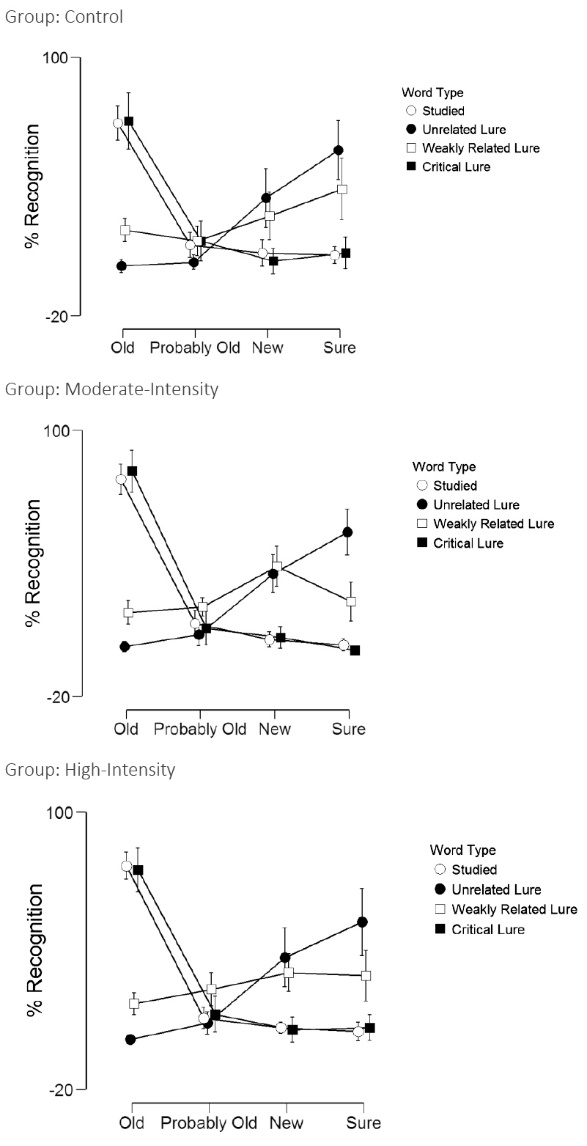
Figure 3. Recognition scores across word type and for each experimental condition.
Discussion
This study evaluated the effects of acute exercise on true episodic memory and false episodic memory. In alignment with previous studies,27,28 with regard to acute exercise and episodic memory, the present experiment demonstrates that acute exercise improves episodic memory. Specifically, the acute high-intensity exercise group recalled significantly more words than the control group for three out of the 6 lists (e.g., Lists 1, 2, and 5). Similarly, the moderate-intensity exercise group recalled significantly more words than the control group for one out of the six lists (e.g., List 2). These results suggest that acute exercise is optimal for enhancing episodic memory and may occur in an intensity-specific fashion. This aligns with our other recent work suggesting that, for episodic memory, high-intensity exercise may be more beneficial than lower-intensity exercise.10 Regarding the false episodic word recall, there was no significant main effect for list, group, or list by group interaction, suggesting that exercise may have a less pronounced effect on false episodic memory recall, when compared to true episodic memory. The memory recognition results showed a significant main effect for recall and a word type by recall interaction. Participants from all three experimental conditions were more likely to perceive the studied and critical lure words as being “old” (i.e., that they previously were exposed to them during the study session).
Similar to the present experiment, our other work by Green et al28 and Siddiqui et al27 also modeled their false episodic memory task after Roediger and McDermott. The high false episodic memory rate was explained by exposure to the semantically related words. Green et al28 hypothesized that exposure to semantically related words may have caused activation of the related lure words, rendering participants to think that the words were previously stated during the encoding task. This explanation could be applied to the results that were found in the present study, as our current experiment also demonstrated a relatively high false episodic memory rate. Taken together, our employed false episodic memory paradigm was robust in inducing false episodic memories.
Limited research has examined the effects of exercise on false episodic memory recall. However, several of our past experimental studies have provided insight into the potential effects of acute exercise on false episodic memory. Siddiqui et al27investigated the time course effects of acute exercise on false episodic memory. We demonstrated that acute exercise prior to the memory task may be optimal in enhancing episodic memory, which is the procedure that the present study utilized. Even though Siddiqui et al27 found no statistically significant results, for their false memory results the study presented evidence to suggest that acute exercise may reduce false episodic memory production. That is, both exercise conditions (before or during encoding) had lower false episodic memory scores when compared to the control condition. Siddiqui et al27 expounded their results with two suggestions. Firstly, that exercise prior to memory encoding may have a priming effect on the neurons, helping prepare them for integration into the memory trace, and overall, creating an optimal environment for true episodic memories.11 Secondly, that moderate-intensity exercise both before and during memory encoding may positively influence executive functioning, an important factor for reducing false episodic memories.11 Our follow-up work by Green et al28 explored the effects of acute exercise on prospective memory and false episodic memory. In the context of the false episodic memory recall results, there was some suggestive evidence that acute exercise reduced the production of false episodic memories.
Although the present experiment did not provide strong evidence of a consistent relationship between exercise intensity and false episodic memory recall, our findings provide some suggestive evidence that higher-intensity exercise may actually increase the likelihood of false episodic memories. This is in contrast to the findings of Green et al28 and Siddiqui et al27 that employed moderate-intensity exercise protocols. These potential intensity-dependent effects may be a result of the effect that acute exercise intensity has on true episodic memory. Higher-intensity acute exercise is more effective in enhancing true episodic memory, and given that our employed false episodic memory paradigm (Roediger and McDermott) involves a critical lure word that is highly semantically related to the studied words, it is plausible that higher-intensity exercise may actually increase the likelihood of false episodic memories, when compared to lower-intensity exercise (such as moderate-intensity exercise). Per the fuzzy trace theory,29 when a memory is encoded, two memory traces are formed, including a verbatim trace and a gist trace, with the latter more likely to decay over time. Speculatively, higher-intensity exercise may be more effective in stabilizing both traces, given the role of higher-intensity exercise on synaptic plasticity. Further, given the role of the prefrontal cortex in inhibiting false episodic memories,30 it is possible that moderate-intensity exercise, which activates the prefrontal cortex,31 may help reduce false episodic memories, whereas high-intensity exercise, which reduces prefrontal cortex activity,32 may accentuate false episodic memories. This is in support of findings form another laboratory that showed that chronic training, in which aerobic fitness was enhanced, increased the ability to correctly identify lure stimuli as similar.33 These assertions align with the accumulating body of research on this topic, including our past two studies by Green et al and Siddiqui et al, along with the present study’s findings.
In addition to false episodic memory, future work should also continue to evaluate whether there is an intensity-specific effect of acute exercise on true episodic memory. As stated, our present findings suggest that higher-intensity exercise is more beneficial for enhancing true episodic memories. This finding aligns with the conclusions of our recent systematic review on this topic.10 However, as noted in our systematic review, very few studies have directly compared different exercise intensities within the same study. The present experiment bridges this gap in the literature by directly comparing control, moderate-intensity and high-intensity exercise protocols. We have discussed these intensity-specific mechanisms in detail elsewhere,7,34 which, in brief, include intensity-specific effects on long-term potentiation. Future work should continue to evaluate whether there is a potential intensity-specific effect of acute exercise on true episodic and false episodic memories.
In conclusion, this study examined the intensity-specific effects of acute exercise on episodic memory and false episodic memory. We did not observe a strong, consistent association between acute exercise and false episodic word recall; however, we did observe evidence to suggest that acute exercise, particularly high-intensity exercise, can improve true episodic memory. Future work on this novel line of inquiry should aim to overcome the limitations of our present experiment. For example, such work should employ a more heterogeneous, representative sample, as well as utilize a within-subject design.
Ethical approval
This study was approved by the University of Mississippi’s ethics committee (#19-003).
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
ED was involved in study conceptualization, data collection and manuscript writing; LZ was involved in manuscript revising; and PL was involved in study conceptualization, statistical analyses and manuscript writing.
References
- Labban JD, Etnier JL. Effects of acute exercise on long-term memory. Res Q Exerc Sport 2011;82(4):712-21. doi: 10.1080/02701367.2011.10599808. [Crossref]
- Johnson MK, Raye CL. Cognitive and brain mechanisms of false memories and beliefs. In: Schacter DL, Scarry E, eds. Memory, brain, and belief. Cambridge, MA: Harvard University Press; 2000:35-86.
- Schnider A, von Daniken C, Gutbrod K. The mechanisms of spontaneous and provoked confabulations. Brain 1996;119 (Pt 4):1365-75. doi: 10.1093/brain/119.4.1365. [Crossref]
- Plancher G, Guyard A, Nicolas S, Piolino P. Mechanisms underlying the production of false memories for famous people’s names in aging and Alzheimer’s disease. Neuropsychologia 2009;47(12):2527-36. doi: 10.1016/j.neuropsychologia.2009.04.026. [Crossref]
- Shapiro BE, Alexander MP, Gardner H, Mercer B. Mechanisms of confabulation. Neurology 1981;31(9):1070-6. doi: 10.1212/wnl.31.9.1070. [Crossref]
- Johnson MK. Source monitoring and memory distortion. Philos Trans R Soc Lond B Biol Sci 1997;352(1362):1733-45. doi: 10.1098/rstb.1997.0156. [Crossref]
- Loprinzi PD, Edwards MK, Frith E. Potential avenues for exercise to activate episodic memory-related pathways: a narrative review. Eur J Neurosci 2017;46(5):2067-77. doi: 10.1111/ejn.13644. [Crossref]
- Chang YK, Tsai CL, Hung TM, So EC, Chen FT, Etnier JL. Effects of acute exercise on executive function: a study with a Tower of London Task. J Sport Exerc Psychol 2011;33(6):847-65.
- Brainerd CJ, Reyna VF. Fuzzy-trace theory and false memory. Curr Dir Psychol Sci 2002;11(5):164-9. doi: 10.1111/1467-8721.00192. [Crossref]
- Loprinzi PD. Intensity-specific effects of acute exercise on human memory function: considerations for the timing of exercise and the type of memory. Health Promot Perspect 2018;8(4):255-62. doi: 10.15171/hpp.2018.36. [Crossref]
- Loprinzi PD, Ponce P, Frith E. Hypothesized mechanisms through which acute exercise influences episodic memory. Physiol Int 2018;105(4):285-97. doi: 10.1556/2060.105.2018.4.28. [Crossref]
- Etnier JL, Wideman L, Labban JD, Piepmeier AT, Pendleton DM, Dvorak KK, et al. The effects of acute exercise on memory and brain-derived neurotrophic factor (BDNF). J Sport Exerc Psychol 2016;38(4):331-40. doi: 10.1123/jsep.2015-0335. [Crossref]
- Frith E, Sng E, Loprinzi PD. Randomized controlled trial evaluating the temporal effects of high-intensity exercise on learning, short-term and long-term memory, and prospective memory. Eur J Neurosci 2017;46(10):2557-64. doi: 10.1111/ejn.13719. [Crossref]
- Jubelt LE, Barr RS, Goff DC, Logvinenko T, Weiss AP, Evins AE. Effects of transdermal nicotine on episodic memory in non-smokers with and without schizophrenia. Psychopharmacology (Berl) 2008;199(1):89-98. doi: 10.1007/s00213-008-1133-8. [Crossref]
- Klaming R, Annese J, Veltman DJ, Comijs HC. Episodic memory function is affected by lifestyle factors: a 14-year follow-up study in an elderly population. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn 2017;24(5):528-42. doi: 10.1080/13825585.2016.1226746. [Crossref]
- Henry JD, Rendell PG. A review of the impact of pregnancy on memory function. J Clin Exp Neuropsychol 2007;29(8):793-803. doi: 10.1080/13803390701612209. [Crossref]
- Sherman SM, Buckley TP, Baena E, Ryan L. Caffeine enhances memory performance in young adults during their non-optimal time of day. Front Psychol 2016;7:1764. doi: 10.3389/fpsyg.2016.01764. [Crossref]
- Bauer EP. Serotonin in fear conditioning processes. Behav Brain Res 2015;277:68-77. doi: 10.1016/j.bbr.2014.07.028. [Crossref]
- Wammes JD, Good TJ, Fernandes MA. Autobiographical and episodic memory deficits in mild traumatic brain injury. Brain Cogn 2017;111:112-26. doi: 10.1016/j.bandc.2016.11.004. [Crossref]
- Hindocha C, Freeman TP, Xia JX, Shaban NDC, Curran HV. Acute memory and psychotomimetic effects of cannabis and tobacco both ‘joint’ and individually: a placebo-controlled trial. Psychol Med 2017;47(15):2708-19. doi: 10.1017/s0033291717001222. [Crossref]
- Le Berre AP, Fama R, Sullivan EV. Executive functions, memory, and social cognitive deficits and recovery in chronic alcoholism: a critical review to inform future research. Alcohol Clin Exp Res 2017;41(8):1432-43. doi: 10.1111/acer.13431. [Crossref]
- McNerney MW, Radvansky GA. Mind racing: The influence of exercise on long-term memory consolidation. Memory 2015;23(8):1140-51. doi: 10.1080/09658211.2014.962545. [Crossref]
- Garber CE, Blissmer B, Deschenes MR, Franklin BA, Lamonte MJ, Lee IM, et al. American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc 2011;43(7):1334-59. doi: 10.1249/MSS.0b013e318213fefb. [Crossref]
- Roediger HL, McDermott KB. Creating false memories: Remebering words not presented in lists. J Exp Psychol Learn Mem Cogn 1995;21(4):803-14. doi: 10.1037/0278-7393.21.4.803. [Crossref]
- Van Calster B, Steyerberg EW, Collins GS, Smits T. Consequences of relying on statistical significance: Some illustrations. Eur J Clin Invest 2018;48(5):e12912. doi: 10.1111/eci.12912. [Crossref]
- The B. Significance testing - are we ready yet to abandon its use? Curr Med Res Opin 2011;27(11):2087-90. doi: 10.1185/03007995.2011.618493. [Crossref]
- Siddiqui A, Loprinzi PD. Experimental investigation of the time course effects of acute exercise on false episodic memory. J Clin Med 2018;7(7). doi: 10.3390/jcm7070157. [Crossref]
- Green D, Loprinzi PD. Experimental effects of acute exercise on prospective memory and false memory. Psychol Rep. 2018:33294118782466. doi: 10.1177/0033294118782466. [Crossref]
- Reyna VF, Brainerd CJ. Fuzzy-trace theory and false memory: new frontiers. J Exp Child Psychol 1998;71(2):194-209. doi: 10.1006/jecp.1998.2472. [Crossref]
- Jeye BM, Karanian JM, Slotnick SD. The anterior prefrontal cortex and the hippocampus are negatively correlated during false memories. Brain Sci 2017;7(1). doi: 10.3390/brainsci7010013. [Crossref]
- Tsujii T, Komatsu K, Sakatani K. Acute effects of physical exercise on prefrontal cortex activity in older adults: a functional near-infrared spectroscopy study. Adv Exp Med Biol 2013;765:293-8. doi: 10.1007/978-1-4614-4989-8_41. [Crossref]
- Ando S, Kokubu M, Yamada Y, Kimura M. Does cerebral oxygenation affect cognitive function during exercise? Eur J Appl Physiol 2011;111(9):1973-82. doi: 10.1007/s00421-011-1827-1. [Crossref]
- Dery N, Pilgrim M, Gibala M, Gillen J, Wojtowicz JM, Macqueen G, et al. Adult hippocampal neurogenesis reduces memory interference in humans: opposing effects of aerobic exercise and depression. Front Neurosci 2013;7:66. doi: 10.3389/fnins.2013.00066. [Crossref]
- Loprinzi PD, Zou L, Li H. The endocannabinoid system as a potential mechanism through which exercise influences episodic memory function. Brain Sci 2019;9(5):E112. doi: 10.3390/brainsci9050112. [Crossref]