Investigating incidence of bacterial and fungal contamination in shared cosmetic kits available in the women beauty salons
Health Promotion Perspectives
eISSN: 2228-6497
Health Promotion Perspectives, 6(3), 159-163; DOI:10.15171/hpp.2016.25
Original Article
Investigating incidence of bacterial and fungal contamination in shared cosmetic kits available in the women beauty salons
Leila Dadashi1,
Reza Dehghanzadeh1,*
1
Department of Environmental Health Engineering, Faculty of Health, Tabriz University of Medical Sciences, Tabriz, Iran
*Corresponding Author: Reza Dehghanzadeh, Department of Environmental Health Engineering, School of Health, Tabriz University of Medical Sciences, Golgasht St., Azadi Ave., Tabriz, Iran. Tel: +98 9144184167; Fax: +98 41 33344731; Email: dehghanzadehr@tbzmed.ac.ir
© 2016 The Author(s). This is an open access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Background: Rich texture of cosmetics can provide a suitable medium for growth of pathogenic microorganisms. In addition, skin microflora of anyone is unique which might be harmful toanother person. Skin and eye pathogenicity could be communicated by sharing cosmetics in beauty saloons. The main objective of this study was to evaluate microbial contamination of in-use skin and eye cosmetics which are available as public make-up kits for women in the beauty salons.
Methods: Fifty-two in-use skin and eye cosmetics were included in this cross sectional study.The specimens from all the cosmetics were collected following the owner’s informed consent,and then about 1 g of the cosmetics was added to nine ml of liquid Eugon LT100 broth medium,two for each product. Ten beauty salons randomly selected from different regions of Tabriz city between June and August 2016. Cosmetics were sampled and carried to the laboratory in sterile condition and then examined to determine bacterial and fungal species in the samples.
Results: All of in-use cosmetic were contaminated with bacteria (95% CI = 93.1%-100.0%) and about 19.2% by fungus and yeast (95% CI = 10.8%-31.9%). Streptococcus spp., Pseudomonas spp., Acinetobacter, Bacillus spp., Staphylococcus spp., Escherichia coli, Salmonella, Klebsiella,Citrobacter, Rhodotorula and Candida were dominant species which were isolated from the cosmetics. Powders with 38.5% (95% CI = 17.7%-64.5%) and eyeliners with 30.0% (95%CI = 6.7%-65.2%) were the most fungal contaminated products.
Conclusion: Shared cosmetics in beauty salons are almost contaminated by bacteria and fungus.Therefore, it is suggested to avoid sharing cosmetics by women and prevent use of public cosmetics in toilet saloons.
Keywords: Shared cosmetic, Beauty salons, Microbial contamination, Women, Bacteria, Fungi
Citation: Dadashi L, Dehghanzadeh R. Investigating incidence of bacterial and fungal contamination in shared cosmetic kits available in thewomen beauty salons. Health Promot Perspect. 2016;6(3):159-163. doi: 10.15171/hpp.2016.25.
Introduction
In recent years, cosmetics are extensively used for beauty purposes. Meanwhile, beauty salons play an important role in possible transfer of skin and eye infections due to the use of public make-up kits by the women.1 Although the microbial standards of cosmetics have been progressively improved by stringent legislations, their contamination has been frequently reported and even in some cases, has generated serious problems for consumers.2 Often production and expiration date are not labeled on the cosmetics, also effectiveness of cosmetic’s preservative decreases with time. In addition, cosmetics comprise essential minerals, growth factors, organic and inorganic compounds and humidity which provide suitable conditions for augmentation of microorganisms.3 Skin microflora of anyone is unique and could be transferred to the others by using common tools such as brushes and pads which could threaten the healthiness of the women.4 Therefore, it is likely that common cosmetics in beauty salons have more diversity and density of microorganisms.
Survey on personal toiletries show that Bacillus, Staphylococcus spp., Pseudomonas spp., Enterobacter, Aspergillus, Penicillium and Candida are more predominant species in cosmetics.3,5 Also the most common skin infections are caused by Staphylococcus epidermis and Staphylococcus aureus.6 However, microbial contamination of in-use cosmetics in beauty salons could be hardly found in the literature and often brushes and combs and other similar devices have been surveyed. The most dominant isolated species from in-use tools in beauty salons have been Streptococcus spp., Staphylococcus spp., Escherichia coli, Citrobacter freundi, Klebsiella, Enterobacter and Pseudomonas aeruginosa and also fungus like Aspergillus and Penicillium.1 Cosmetic products can be contaminated by three ways; (1) application of unsterile raw material as ingredients, (2) in the course of production process, or (3) during use of cosmetics.7 On the other hand, trafficking counterfeit cosmetic products is the serious problem in many countries.
Consumption of cosmetics is growing in developing countries. Microbial contamination and occurrence of skin contamination due to cosmetics is still one of the major causes for product recalls in the world.8 Then, the main purpose of this study was to evaluate bacterial and fungal contamination of in-use eye and skin cosmetics shared by women in beauty salons.
Materials and Methods
Sampling
To determine the microbial contamination of in-use shared cosmetics available in the beauty salons, about 52 in-use skin (powder and cream) and eye (mascara and eyeliner) cosmetics were included in this cross-sectional study based on sample size calculation for dichotomous variable. The sample size was estimated about 61 (significance level = 0.05, population proportion = 0.2 and relative error = 10%), but nine of the samples were discarded because of probably contamination during handling. The specimens from all the cosmetics were collected following the owner’s informed consent, and then about one gram of the cosmetics was added to nine ml of liquid Eugon LT100 broth medium, two for each product. Ten beauty salons randomly selected from different reign of Tabriz city between June and August 2016. Sampling of cosmetics was conducted in the salons.
Microbial survey
In sterile conditions, about 1 g of the cosmetics was added to nine ml of liquid Eugon LT100 broth medium to neutralize the growth inhibitors present in the ingredients of the cosmetics. The samples immediately were carried to the laboratory and analyzed in accordance with the standards of Food and Drug Administration (FDA) and Institute of Standards and Industrial Research of Iran.9 First the tubes were incubated for 48-72 hours at 37°C. Then, 1 mL of each culture was removed and transferred to the Cetrimide Agar medium, Levine eosin methylene blue Agar medium, Baird Parker Agar, and Sabouraud Dextrose Chloramphenicol Agar and incubated for 24-48 hours at 37°C. Afterwards, the plates containing growing colonies were isolated and the total count of colony forming unit per gram or milliliter of cosmetics (CFU g-1) was determined by counting the colonies on the medias. Further identification of the isolated bacteria were carried out according to the bacteria’s morphology and biochemical tests using standard bacteriological methods.10 Fungi and molds were identified in terms of appearance. In addition, the relevant test to detecting Candida yeast including culturing in human serum and incubation at 37°C was conducted for 3 hours.11
Statistical analyses
Variance between the contamination levels in the in-use cosmetics as well as between different cosmetic types was determined by chi-square k-sample Pearson analysis with significance level of 0.05 using SPSS software (IBM SPSS Statistics 19, SPSS Inc., USA). Confidence intervals (CI) were calculated by Stata MP 14 (Stata Corp LP, USA).
Results
Table 1 shows that, exactly 100% (95% CI = 93.1%-100%) of the total examined in-use cosmetics in the beauty salons were contaminated by bacteria. However, only 19.2% (95% CI = 10.8%-31.9%) of the cosmetic products were contaminated by fungi or yeast. Generally powders demonstrated higher contamination by fungi. The results show that creams did not indicated any contamination by fungi.
|
Table 1. Summery of microbial contamination rate in the sampled cosmetics from women beauty salons
|
|
Cosmetic type
|
No. of samples
|
Microbial contamination rate
|
|
|
Bacteria
|
Fungi
|
|
|
n (%)
|
95% CI
|
P value
|
n (%)
|
95% CI
|
P value
|
| Skin |
|
|
|
|
|
|
|
| Powder |
13 |
13(100.0 ) |
93.12-100.0 |
NSa |
5(38.5) |
17.7-64.5 |
|
| Cream powder |
12 |
12(100.0) |
75.8-100.0 |
NS |
0.0 |
0.0-24.3 |
|
| Eye |
|
|
|
|
|
|
0.063 |
| Mascara |
17 |
17(100.0) |
81.6-100.0 |
NS |
2(11.8) |
3.3-34.3 |
|
| Eyeliner |
10 |
10(100.0) |
72.5-100.0 |
NS |
3(30.0) |
10.8-60.3 |
|
|
a Because of complete response, no significant diffeence was observed between cosmetic types.
|
The number of colony forming units of fungi in cosmetics was between 3.5-200×103 CFU g-1 (Table 2). Also the number of colony forming units of isolated bacteria was 12-960×103 CFU g-1. High levels of Staphylococcus spp. and Escherichia coli counts (>500 CFU g-1) were found in the in-use powders and eyeliners.
|
Table 2. Microbial Counts (103 CFU g-1) and association between contamination by bacteria and fungi in shared cosmetics available in women beauty salons
|
|
Microorganisms
|
Powder
|
Cream
|
Mascara
|
Eyeliner
|
P
value
|
| Bacteria |
|
|
|
|
|
|
Acinetobacter
|
300 |
350 |
320 |
NC |
0.255 |
|
Escherichia coli
|
NCa |
- |
- |
850 |
0.008 |
|
Bacillus
|
NC |
230 |
320 |
500 |
0.802 |
|
Pseudomonas
|
208 |
195 |
180 |
125 |
0.576 |
|
Staphylococcus
|
960 |
544 |
410 |
144 |
0.518 |
|
Streptococci
|
NC |
23 |
440 |
684 |
0.324 |
|
Klebsiella
|
- |
- |
21 |
- |
0.233 |
|
Citrobacter
|
- |
- |
12 |
- |
0.552 |
|
Salmonella
|
- |
- |
32 |
- |
0.233 |
|
Alcanigenes
|
- |
- |
20 |
- |
0.383 |
| Fungi |
|
|
|
|
|
|
Candida
|
- |
- |
30 |
- |
0.662 |
|
Rhodotorula
|
200 |
- |
126 |
115 |
0.131 |
|
Penicillium
|
3.5 |
- |
- |
- |
0.100 |
|
aNon-countable.
|
Figure 1 and 2 demonstrate the diversity and frequency of the isolated bacteria and fungi separately in skin and eye cosmetics obtained from beauty salons. Fungi and bacteria constituted 9.2% (95% CI = 5.1%-16.1%) and 90.8% (95% CI = 83.9%-94.9%) of the isolates, respectively. Also about 51.5% (95% CI = 41.8%-61.1%) of the isolated bacteria were belong to gram-negative group and the remains were gram-positive. Streptococcus spp., Acinetobacter and Pseudomonas spp. were the most dominant in the skin cosmetics. Candida, Rhodotorula and Penicillium were the only isolated yeasts and fungi. Also Bacillus spp., Staphylococcus spp. and Escherichia coli isolated from the skin cosmetics. Streptococcus spp., Pseudomonas spp. and Acinetobacter were the most frequently isolated bacteria from in-use eye cosmetics. Rhodotorula and candida were the only isolated yeasts. Also Bacillus spp, Staphylococcus spp., Escherichia coli, Salmonella, Klebsiella and Citrobacter were isolated from the eye cosmetics.
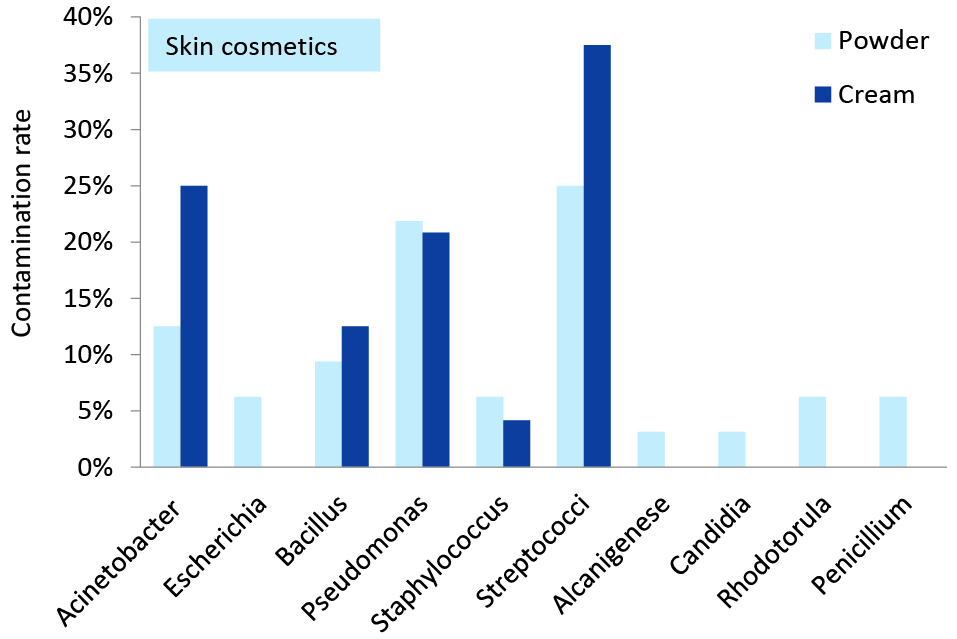
Figure 1. Microbial contamination rate in the in-use skin cosmetics in women beauty salons.
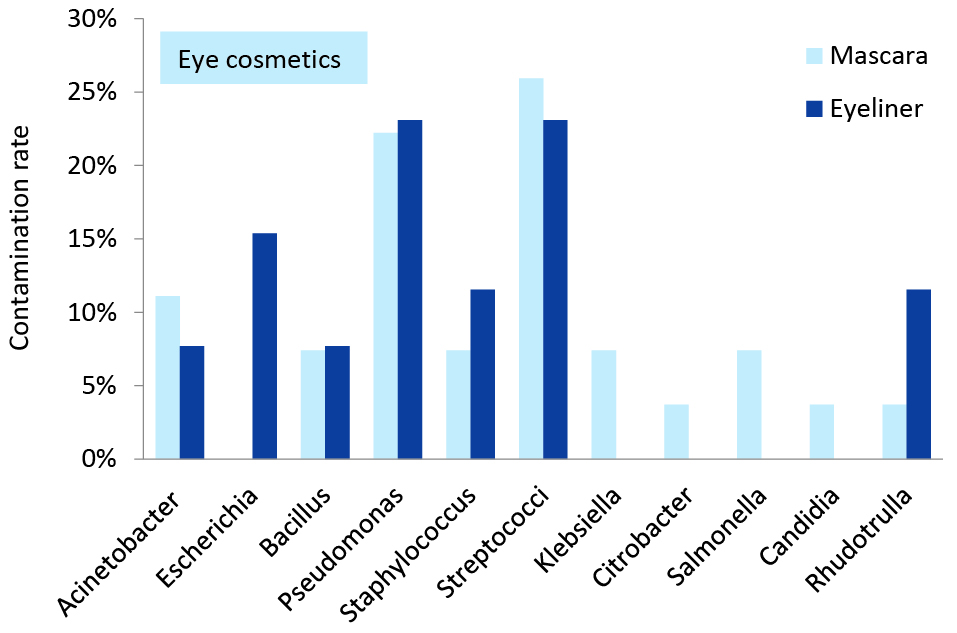
Figure 2. Microbial contamination rate in the in-use eye cosmetics in women beauty salons.
Streptococcus species was the most predominant bacteria that were isolated from the in-use skin and eye cosmetics. Considering the fungi isolated from in-use skin powders, Penicillium was definitely the most predominant fungi genus (6%), which was followed in order by Rhodotorula (6%) and Candida (3%). Furthermore, Rhodotorula (12%) and Candida (4%) were the most isolated fungi from the in-use mascaras and eyeliners. Moreover, the most fungal diversity was observed in the in-use skin powders. Referring to the isolated bacteria from the in-use skin cosmetics, the most predominant bacteria were Streptococcus (32%), Pseudomonas (23%), Acinetobacter (19%), Bacillus (11%), Staphylococcus (6%) and E. coli (4%). Among the in-use eye cosmetics, Streptococcus (25%) and Pseudomonas (24%) were the predominant isolated bacteria, which was followed in a descending order by Acinetobacter and Staphylococcus (10% each), Bacillus and E. coli (8% each), Salmonella and Klebsiella (4% each), and Citrobacter (2%).
Discussion
Results showed that all of the sampled cosmetics were contaminated by bacteria which is more than the rate of 63% reported from in-use individual cosmetics.8 Preservatives of the cosmetics remain active on the skin that might alter the skin microflora which are responsible for protection and supplying skin safety.12 Cosmetics are not produced in sterile condition and are often shared in beauty salons which could cause the increase of microbial contamination within cosmetics.13 Contamination level in our study is higher in comparison with a study in the United States that was conducted on 3000 shared cosmetic tester kits available to the public and reported 50% contamination of the products by bacteria.14
Contamination level in the powders was higher than the other cosmetics. It can be deduced that the powders are frequently in contact with air and also the common use of skin powder pads can cause the higher contamination rate. In addition, application of the natural ingredients in the formulation of powders including talc, Fuller’s earth and bentonite might increase the contamination level.15 Contaminated eye cosmetics, particularly mascaras, are associated with ocular infections.5,16 Our result revealed that in all the examined cosmetics, mascaras had a more bacterial diversity, because of its aqueous-based formulation and greater chance of bacterial deposits originating from the environment and from the surface of the eyelashes, which makes the product more susceptible to infections.6,13
About 19.2% (95% CI = 10.8%-31.9%) of in-use cosmetics were contaminated by fungus and yeast. Fungus contamination ratio in cosmetics was low compared with bacteria. It can be attributed to the more ability of cosmetics preservatives in prevention of fungus growth.17 In this study, isolation of gram-negative bacteria was more than gram-positive bacteria, while gram-positive bacteria is more predominant in the skin flora.17,18 It can be concluded by the more resistance of gram-negative bacteria to severe condition which could cause growth of them in cosmetics. Streptococci species were the most dominant isolated bacteria, which also have been reported in personal cosmetics.19 Streptococcus species can cause skin infections like Erythematous rash.20 Pseudomonas spp. was the most dominant species isolated from the eyeliners. Pseudomonas aeruginosa have been mostly reported in personal cosmetics.2,5,21 Because Pseudomonas aeruginosa is one of the natural skin microflora , it can be transferred to the cosmetics from consumer’s skin.18 Pseudomonas aeruginosa can cause skin infections.22 Also, Acinetobacter was isolated from the examined cosmetic and as skin microflora play an important role in skin infections.18,23 Bacillus spp., Staphylococcus spp. and Escherichia coli were the other isolated bacteria from the cosmetic. Staphylococcus spp. causes skin infections such as acne and desquamate.24 Bacillus species are transient skin microflora. Bacillus anthracis causes focal necrotizing cellulitis in the skin. Use of eye cosmetics contaminated with Bacillus cereus causes severe eye infections.24 Candida and Rhodotorula also were isolated from the cosmetics. Candida has been reported in other personal toiletries studies.5,25 Candida plays an important role in the establishment of skin lesions, rash and dermatitis.26 Also in this study Salmonella, Citrobacter, Klebsiella and Alcanigenes have been isolated.
Higher density and diversity of bacteria isolated from shared cosmetics that obtained from beauty salons in comparison with personal cosmetics reported in the literatures.3,5,21,27 When several people share the same cosmetic an instance contamination may take place and because each individual has unique skin microflora that could be harmful to another person. The number of colony forming units of aerobic mesophilic microorganisms for eye and other cosmetics must not exceed 102 and 103 CFU g-1 of products, respectively.28 In all the examined cosmetics available for public make-up in women beauty salons, the number of microbial counts was more than the maximum favorable level.
Conclusion
Finally, our findings showed that microbial contamination rate in cosmetics which are shared in beauty salons is higher than the rates reported for personal cosmetics in the literatures. However, the hygienic condition of the salons, socioeconomic level of the area that the beauty salons are located and even individual health behaviors of the costumers could be impressive factors on the contamination rate of cosmetics which are not assessed in this study.
The skin microflora of anyone is distinctive and when several people share the same product, the rate of contamination could be increased. Therefore, it is suggested to avoid long-term use, share or use public cosmetics in toilet saloons and keep the used cosmetics in dry, cool, and fastened packets. Also, it is necessary to promote or make compulsory the use of individual cosmetic kits in the beauty salons, intensify the hygiene inspections from the beauty salons, monitor behavior of the barbers and implement continuous health education programs by the hygiene inspectors for the beauty salon workers.
Acknowledgments
This study is part of an MSc thesis in Environmental Health Engineering, which was submitted to Tabriz University of Medical Sciences (5/53/4643-1393.08.14). We appreciate Research Vice-Chancellor of Tabriz University of Medical Sciences for their financial support. We thank all the beauty salon lords who participated in sampling of their cosmetic kits.
Ethical approval
Procedures were approved by the Research Vice-Chancellor of Tabriz University of Medical Sciences review board.
Competing interests
No conflict of financial and non-financial interest declared between the authors.
Authors’ contributions
LD was involved in the conception of the study, performed data collection and the analyses and drafted the manuscript. RD was involved in the conception of the study, interpreted the results from the analyses, performed significant revisions, assisted in the revision of the manuscript and approved the final version of the manuscript.
References
- Enemuor S, Ojih M, Isah S, Oguntibeju O. Evaluation of bacterial and fungal contamination in hairdressing and beauty salons. Afr J Microbiol Res 2013;7(14):1222-5. doi: 10.5897/AJMR12.917. [Crossref]
- Lundov M, Moesby L, Zachariae C, Johansen J. Contamination versus preservation of cosmetics: a review on legislation, usage, infections, and contact allergy. Contact Dermatitis. 2009(60):70-8. doi: 10.1111/j.1600-0536.2008.01501.x.
- Behravan J, Bazzaz F, Malaekeh P. Survey of bacteriological contamination of cosmetic creams in Iran (2000). Int J Dermatol 2005;44(6):482-5. doi: 10.1111/j.1365-4632.2005.01963.x. [Crossref]
- Noah N. A guide to hygienic skin piercing. In: Gerson J, ed. Milady’s Standard Textbook for Professional Estheticians. New York: Milady; 1995. pp. 1-11.
- Anelich L, Korsten L. Survey of micro‐organisms associated with spoilage of cosmetic creams manufactured in South Africa. Int J Cosmetic Sci 1996;18(1):25-40. doi: 10.1111/j.1467-2494.1996.tb00133.x. [Crossref]
- Draelos ZD. Special considerations in eye cosmetics. Clin Dermatol 2001;19(4):424-30. doi: 10.1016/S0738-081X(01)00204-8. [Crossref]
- Charnock C. The microbial content of nonsterile pharmaceuticals distributed in Norway. J Hosp Infect 2004;3(57):233-40. doi: 10.1016/j.jhin.2004.03.016. [Crossref]
- Okeke I, Lamikanra A. Bacteriological quality of skin-moisturizing creams and lotions distributed in a tropical developing country. J Appl Microbiol 2001;91(5):922-8. doi: 10.1046/j.1365-2672.2001.01456.x. [Crossref]
- Microbiology of cosmetics: Detection of specified and non-specified microorganisms. Tehran: Institute of Standards and Industrial Research of Iran; 1992.
- Vos P, Garrity G, Jones D, Krieg NR, Ludwig W, Rainey FA, et al. Springer Science & Business Media; 2011. Bergey’s Manual of Systematic Bacteriology. The Firmicutes, vol 3.
- Williams DW, Lewis MA. Oral Microbiology: Isolation and identification of candida from the oral cavity. Oral Dis 2000;6(1):3-11. doi: 10.1111/j.1601-0825.2000.tb00314.x. [Crossref]
- Holland KT, Bojar RA. Cosmetics. Am J Clin Dermatol 2002;3(7):445-9. doi: 10.2165/00128071-200203070-00001. [Crossref]
- Giacomel C, Dartora G, Dienfethaeler H, Haas S. Investigation on the use of expired make-up and microbiological contamination of mascaras. Int J Cosmetic Sci. 2013:375-80. doi: 10.1111/ics.12053.
- Tran TT, Hitchins AD. Microbial survey of shared-use cosmetic test kits available to the public. J Ind Microbiol 1994;13(6):389-91. doi: 10.1007/BF01577224. [Crossref]
- Kulkarni SB, Bajpai ND, Meghre VS. Evaluation of dome marketed facepacks and cakes for microbial load. Asian J Microbiol Biotechnol Environ Sci 2011;13(1):213-6.
- Kabara JJ. Preservative-Free and Self-preserving Cosmetics and Drugs: Principles and Practices. Boca Raton, FL: CRC Press; 1997.
- Mislivec P, Bandler R, Allen G. Incidence of fungi in shared-use cosmetics available to the public. J AOAC Int 1992;76(2):430-6.
- Bojar R, Holland K. Review: the human cutaneous microflora and factors controlling colonisation. World J Microb Biot 2002;18(9):889-903. doi: 10.1023/A:1021271028979. [Crossref]
- Pack LD, Wickham MG, Enloe RA, Hill DN. Microbial contamination associated with mascara use. J Am Optom Assoc 2008;79(10):587-93. doi: 10.1016/j.optm.2008.02.011. [Crossref]
- Kolmos H, Svendsen R, Nielsen S. The surgical team as a source of postoperative wound infections caused by Streptococcus pyogenes. J Hosp Infect 1997;35(3):207-14. doi: 10.1016/S0195-6701(97)90208-5. [Crossref]
- Wong S, Street D, Delgado SI. Recalls of foods and cosmetics due to microbial contamination reported to the US Food and Drug Administration. J Food Protect 2000;63(8):1113-6.
- Murthy R, Sengupta S, Maya N, Shivananda P. Incidence of post operative wound infection and their antibiogram in a teaching and referral hospital. Indian J Med Sci 1998;52(12):553-5.
- Jones ME, Karlowsky JA, Draghi DC, Thornsberry C, Sahm DF, Nathwani D. Epidemiology and antibiotic susceptibility of bacteria causing skin and soft tissue infections in the USA and Europe: a guide to appropriate antimicrobial therapy. Int J Antimicrob Agents 2003;22(4):406-19. doi: 10.1016/S0924-8579(03)00154-7. [Crossref]
- Leyden JJ, Kligman AM. Acne vulgaris: new concepts in pathogenesis and treatment. Drugs 1976;12(4):292-300. doi: 10.2165/00003495-197612040-00004. [Crossref]
- Lundov MD, Zachariae C. Recalls of microbiologically contaminated cosmetics in EU from 2005 to May 2008. Int J Cosmetic Sci 2008;30(6):471-4. doi: 10.1111/j.1468-2494.2008.00475.x. [Crossref]
- Rebora A, Leyden J. Napkin (diaper) dermatitis and gastrointestinal carriage of Candida albicans. Brit J Dermatol 1981;105(5):551-5. doi: 10.1111/j.1365-2133.1981.tb00798.x. [Crossref]
- Baird R. Bacteriological contamination of products used for the skin care in babies. Int J Cosmet Sci 1984;6(2)85-90. doi: 10.1111/j.1467-2494.1984.tb00362.x. [Crossref]
- Scientific Committee on Consumer Products. The SCCP’s Notes of Guidance for the Testing of Cosmetic Ingredients and Their Safety Evaluation. 2006. Available from: http://ec.europa.eu/health/scientific_committees/consumer_safety/docs/sccs_s_006.pdf.